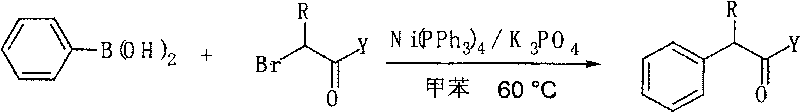Method for preparing alpha-aryl carbonyl compound
A carbonyl compound and arylcarbonyl technology, applied in the field of preparation of α-aryl carbonyl compounds, can solve the problems of inability to contain β hydrogen and narrow substrate range, and achieve the effects of mild reaction conditions, easy preparation and stable substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Reaction of 2-Bromoesters or Amides
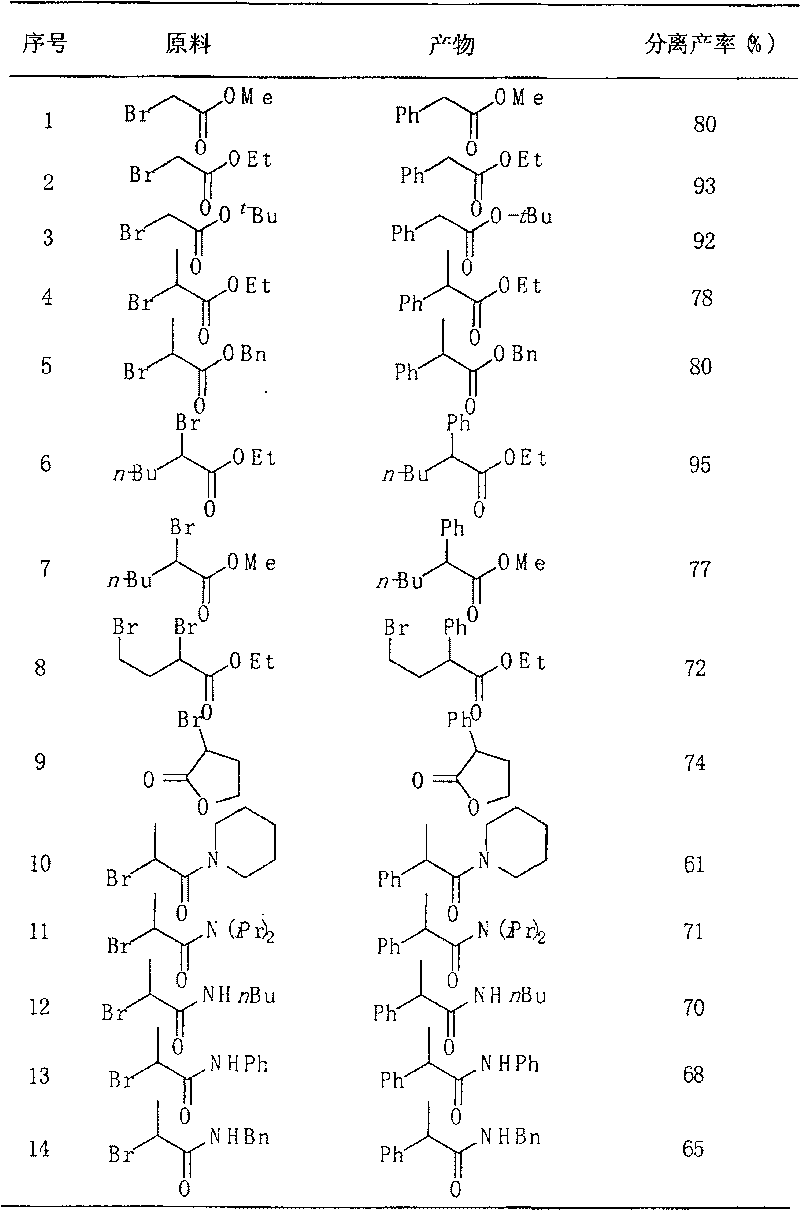
[0017] In a reaction system, add phenylboronic acid (243.8mg, 2.0mmol), 2-bromo ester or amide (1.0mmol), Ni(PPh 3 ) 4 (110.8 mg, 0.1 mmol), K 3 PO 4 (849.2 mg, 4.0 mmol), the system was replaced by nitrogen protection, 2 ml of toluene was added, and the reaction was carried out at 60 degrees Celsius for 16 hours. Stop the reaction, add 2 ml of 2M dilute hydrochloric acid to the system, add 5-10 ml of ethyl acetate three times for extraction, combine the organic phases, and dry over anhydrous sodium sulfate. The product 2-phenyl ester or amide was obtained by column chromatography, and the separation yield is shown in Table 1 below.
[0018]
[0019] Table 1 Arylation reaction of phenylboronic acid with 2-bromoester and amide
[0020]
Embodiment 2
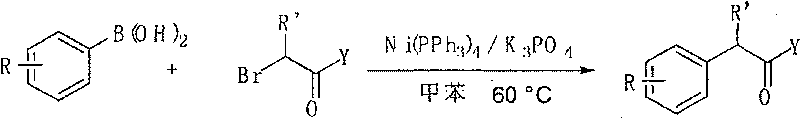
[0022] In a reaction system, add substituted phenylboronic acid (2.0mmol), 2-bromo ester or amide (1.0mmol), Ni(PPh 3 ) 4 (110.8 mg, 0.1 mmol), K 3 PO 4 (849.2 mg, 4.0 mmol), the system was replaced by nitrogen protection, 2 ml of toluene was added, and the reaction was carried out at 60 degrees Celsius for 16 hours. Stop the reaction, add 2 ml of 2M dilute hydrochloric acid to the system, add 5-10 ml of ethyl acetate three times for extraction, combine the organic phases, and dry over anhydrous sodium sulfate. The product 2-substituted phenyl ester or amide was obtained by column chromatography, and the separation yield is shown in Table 2 below.
[0023]
[0024] Table 2 Reaction of substituted phenylboronic acids with 2-bromo esters and amides
[0025]
[0026] b The reaction temperature is 80 degrees Celsius
Embodiment 3
[0027] Embodiment 3: the reaction of 2-bromoketone or 2-chloroketone
[0028] In a reaction system, add arylboronic acid (2.0mmol), 2-bromoketone or 2-chloroketone (1.0mmol), Ni(PPh 3 ) 4 (110.8 mg, 0.1 mmol), K 3 PO 4 (1273.8 mg, 6.0 mmol), the system was replaced with nitrogen protection, 2 ml of toluene was added, and the reaction was carried out at 60 degrees Celsius for 16 hours. Stop the reaction, add 2 ml of 2M dilute hydrochloric acid to the system, add 5-10 ml of ethyl acetate three times for extraction, combine the organic phases, and dry over anhydrous sodium sulfate. 2-aryl ketone was obtained by column chromatography. The separation yield is shown in Table 3 below.
[0029]
[0030] Table 3: Nickel-catalyzed arylation of ketones
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com