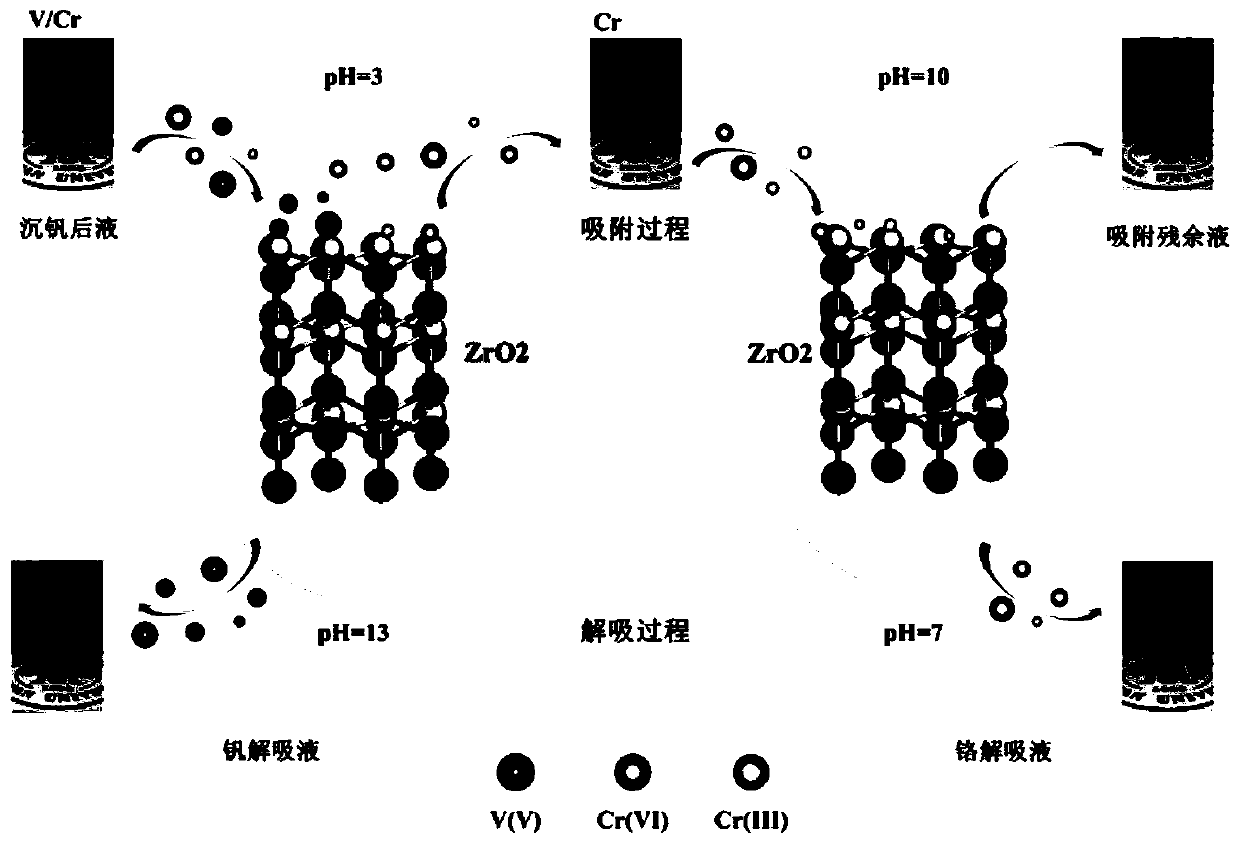
Adsorption method for separating and extracting vanadium and chrome from solutions after vanadium precipitation
A technology of solid-liquid separation and vanadium precipitation, which is applied in chemical instruments and methods, adsorption water/sewage treatment, and process efficiency improvement, etc. Friendly, easy-to-prepare effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1- Embodiment 3
[0041] Preparation of embodiment 1-embodiment 3 zirconia adsorbent
[0042] Embodiment 1-Example 3 prepares zirconia adsorbent according to the preparation process conditions given in Table 1 through the following steps:
[0043] (S1) Concentrated sulfuric acid, the first organic solvent and zirconium n-butoxide are uniformly mixed according to the ratio given in Table 1 to prepare solution A;
[0044] (S2) Mix the second organic solvent and deionized water according to the ratio given in Table 1 to prepare solution B;
[0045] (S3) adding solution B dropwise to solution A to obtain a solid colloid, which is cured and dried at 80° C. to obtain a zirconia precursor;
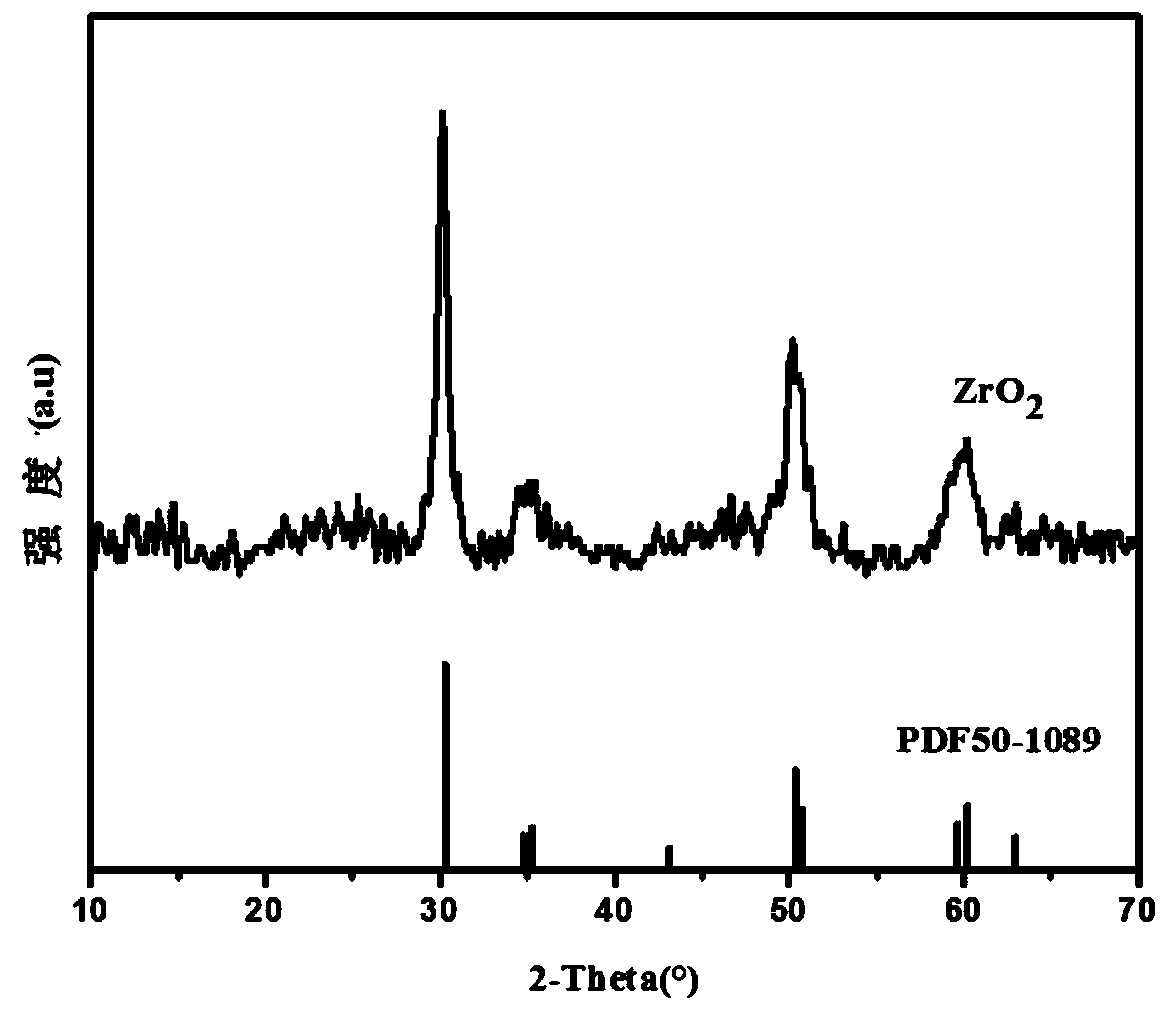
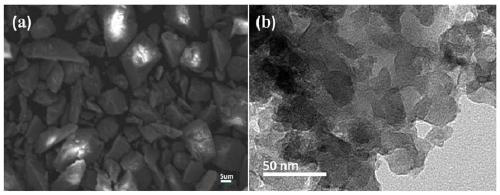
[0046] (S4) Calcining the obtained zirconia precursor at 500-600°C for 3-6 hours under air ventilation conditions to obtain massive zirconia, which is ground and sieved (through 100-mesh and 200-mesh sieves in sequence) That is, the zirconia adsorbent is obtained.
[0047] Embodiment 1-Example 3 Zirconia adsorb...
Embodiment 4
[0069] Example 4 Separation and enrichment of vanadium and chromium in the liquid after vanadium precipitation
[0070] In this example, the zirconia prepared in Example 2 was used as the adsorbent.
[0071] (1) Adsorption of vanadium from the solution after vanadium precipitation
[0072] To the first container filled with the solution after vanadium precipitation (the concentration of vanadium is 100.7mg / L, and the concentration of chromium is 99.07mg / L), adding nitric acid with a concentration of 1M to adjust the pH value of the solution after vanadium precipitation to 3, and then according to the zirconia The ratio of the mass of the adsorbent to the volume of the vanadium-precipitated liquid is 0.8g / L. Put the zirconia adsorbent into the vanadium-precipitated liquid after adjusting the pH value for vanadium adsorption. Zirconia adsorbent with vanadium (V-ZrO 2 ) and the liquid after primary adsorption;
[0073] (2) Adsorption of chromium from the liquid after primary a...
Embodiment 5
[0077] Example 5 Separation and enrichment of vanadium and chromium in the solution after vanadium precipitation
[0078] In this example, the zirconia prepared in Example 2 was used as the adsorbent.
[0079] (1) Adsorption of vanadium from the solution after vanadium precipitation
[0080] In the first container filled with the vanadium-precipitated liquid (the vanadium concentration is 100.7mg / L, the chromium concentration is 99.07mg / L), adding the hydrochloric acid with a concentration of 1M to adjust the pH value of the vanadium-precipitated liquid to 3, and then according to the zirconia The ratio of the mass of the adsorbent to the volume of the vanadium-precipitated liquid is 0.8g / L. Put the zirconia adsorbent into the vanadium-precipitated liquid after adjusting the pH value for vanadium adsorption. Zirconia adsorbent with vanadium (V-ZrO 2 ) and the liquid after primary adsorption;
[0081] (2) Adsorption of chromium from the liquid after primary adsorption
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com