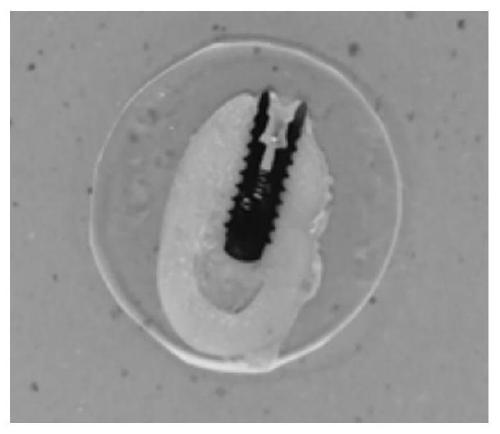
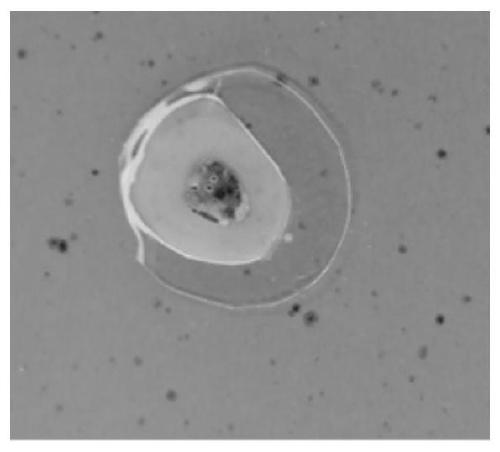
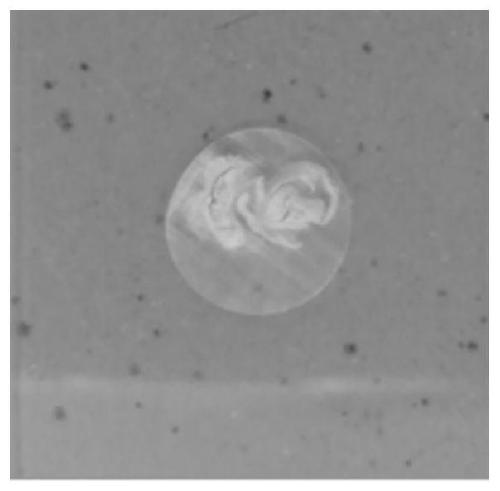
Tissue embedding solution and tissue slice preparation method
A technology for tissue sectioning and embedding fluid, which is applied in the field of pathological analysis and can solve problems such as insufficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] 1. Fix the bone tissue containing the metal implant in 10% formaldehyde solution by volume for 72 hours at room temperature;
[0069] 2. At normal temperature, dehydrate the sample obtained in step 1 in the ethanol aqueous solution with the volume fraction of 70%, 80%, 90%, 95%, 100% and 100% successively, and the dehydration time of each stage is 3 Hour;
[0070] 3. At room temperature, make the sample obtained in step 2 transparent twice in xylene, and the transparent time is 4 hours each time;
[0071] 4. At 0°C, use the first-stage permeate, the second-stage permeate, and the third-stage permeate to perform step-by-step infiltration treatment on the sample obtained in step 3, wherein the first-stage permeate includes formazan by volume ratio Hydroxyethyl acrylate 30mL, methyl methacrylate 55mL, butyl methacrylate 10mL and polyethylene glycol 400 5mL. The second-stage permeate is the same as the third-stage permeate, including that the first-stage permeate includes...
Embodiment 2
[0076] 1. Fix the bone tissue in 10% by volume formaldehyde solution for 24 hours at room temperature;
[0077] 2. At normal temperature, dehydrate the sample obtained in step 1 in the ethanol aqueous solution with the volume fraction of 70%, 80%, 90%, 95%, 100% and 100% successively, and the dehydration time of each stage is 3 Hour;
[0078] 3. At room temperature, make the sample obtained in step 2 transparent twice in xylene, and the transparent time is 4 hours each time;
[0079] 4. At 4°C, use the first-stage permeate, the second-stage permeate, and the third-stage permeate to sequentially perform step-by-step infiltration treatment on the sample obtained in step 3, wherein the first-stage permeate includes formazan by volume ratio. Hydroxyethyl acrylate 30mL, methyl methacrylate 56mL, butyl methacrylate 7mL and polyethylene glycol 4007mL. The second-stage permeate is the same as the third-stage permeate, including the first-stage permeate including 30 mL of hydroxyethy...
Embodiment 3
[0084] 1. Fix the bone tissue in 10% formaldehyde solution by volume for 12 hours at room temperature;
[0085] 2. At room temperature, dehydrate the samples obtained in step 1 in the ethanol aqueous solution with volume fractions of 70%, 80%, 90%, 95%, 100% and 100% successively, and the dehydration time of each stage is 6 Hour;
[0086] 3. At room temperature, make the sample obtained in step 2 transparent twice in xylene, and the transparent time is 6 hours each time;
[0087]4. At 2°C, use the first-stage permeate, the second-stage permeate, and the third-stage permeate to sequentially perform step-by-step infiltration treatment on the sample obtained in step 3, wherein the first-stage permeate includes formazan by volume ratio Hydroxyethyl acrylate 35mL, methyl methacrylate 55mL, butyl methacrylate 5mL and polyethylene glycol 4005mL. The second-stage permeate is the same as the third-stage permeate, including that the first-stage permeate includes 35 mL of hydroxyethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com