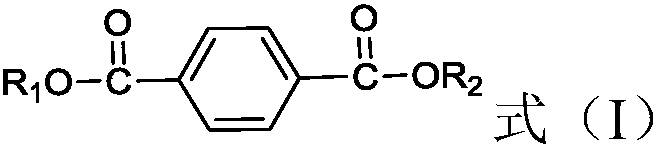
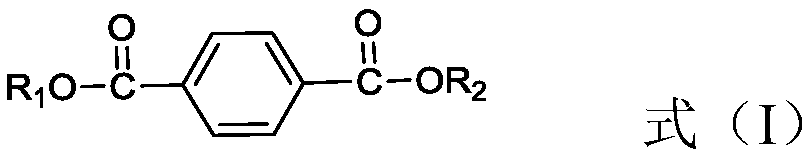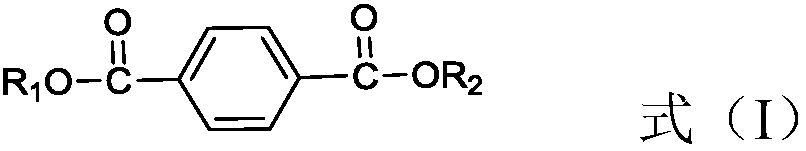Method used for producing 1, 4-cyclohexanedimethanol
A technology of cyclohexanedimethanol and cyclohexanedicarboxylate, which is applied in the field of producing 1,4-cyclohexanedimethanol, can solve the problems of low solubility and high melting point of dimethyl terephthalate, and achieve The effect of low raw material residue, good product quality, and reduced hydrogen consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Primary hydrogenation unit: Dioctyl terephthalate and H 2 Pass through the fixed bed reactor together from bottom to top (the catalyst packed in it is Pd-Rh / Al 2 o 3 (0.3% by weight Pd, 0.3% by weight Rh), the catalyst in H 2 Atmosphere, reduction and activation at 150°C for 6 hours) for hydrogenation reaction, a micro-nanoporous membrane (the average diameter of nanopores is 20nm, and the average diameter of micropores is 800μm) is set in front of the fixed-bed reactor, and hydrogen passes through the micro-nanoporous membrane , highly dispersed or dissolved in dioctyl terephthalate. The hydrogenation reaction conditions are: temperature is 110°C, pressure is 3MPa, H 2 The molar ratio to dioctyl terephthalate is 10, and the weight space velocity of dioctyl terephthalate is 0.9h -1 . According to calculation, the conversion rate of dioctyl terephthalate at the outlet of the primary hydrogenation unit is 99.9%, and the selectivity of dioctyl 1,4-cyclohexanedicarboxy...
Embodiment 2
[0045] Primary hydrogenation unit: Dibutyl terephthalate and H 2 Pass through the fixed bed reactor together (the loaded catalyst is Ru / SiO 2 (2.3% by weight Ru), the catalyst in H 2 atmosphere, reduction and activation at 120°C for 10 hours) for hydrogenation reaction, the hydrogenation reaction conditions are: temperature is 60°C, pressure is 10MPa, H 2 The molar ratio to dibutyl terephthalate is 3.5, and the weight space velocity of dibutyl terephthalate is 1.3h -1 . It is calculated that the conversion rate of dibutyl terephthalate at the outlet of the primary hydrogenation unit is 99.8%, and the selectivity of dibutyl 1,4-cyclohexanedicarboxylate is 100%.
[0046] Secondary hydrogenation unit: the primary hydrogenation product containing dibutyl 1,4-cyclohexanedicarboxylate obtained from the primary hydrogenation unit and H 2 Pass through the fixed bed reactor together (the loaded catalyst is CuO-MnO 2 -ZrO 2 (60% by weight CuO, 35% by weight MnO 2 , 5% by weight Z...
Embodiment 3
[0049] Primary hydrogenation unit: Dioctyl terephthalate and H 2 Pass through fixed bed reactor together (the catalyst of packing is Rh-Pd / C (0.3% by weight Rh, 0.2% by weight of Pd), described catalyst is in H 2 atmosphere, reduction and activation at 180°C for 5 hours) for hydrogenation reaction, the reaction conditions are: temperature 160°C, pressure 0.1MPa, H 2 The molar ratio to dioctyl terephthalate is 100, and the weight space velocity of dioctyl terephthalate is 3h -1 . According to calculation, the conversion rate of dioctyl terephthalate at the outlet of the primary hydrogenation reactor is 99.6%, and the selectivity of dioctyl 1,4-cyclohexanedicarboxylate is 99.1%.
[0050] Secondary hydrogenation unit: the primary hydrogenation product containing dioctyl 1,4-cyclohexanedicarboxylate obtained from the primary hydrogenation unit and H 2 Pass through the fixed bed reactor together (the loaded catalyst is CuO-ZnO-Al 2 o 3 (42% by weight CuO, 38% by weight ZnO, 20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com