Olefinated ethinyloestradiol compound, and preparation and application thereof
A compound, the technology of ethinyl estradiol, which is applied in the fields of olefinated ethinyl estradiol compounds and its preparation and application, can solve the long-term, high morbidity and mortality of diseases, and the correlation between occurrence and progress has not been well understood. Interpretation and other issues to achieve the effect of novel structure, excellent inhibitory activity, and good antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
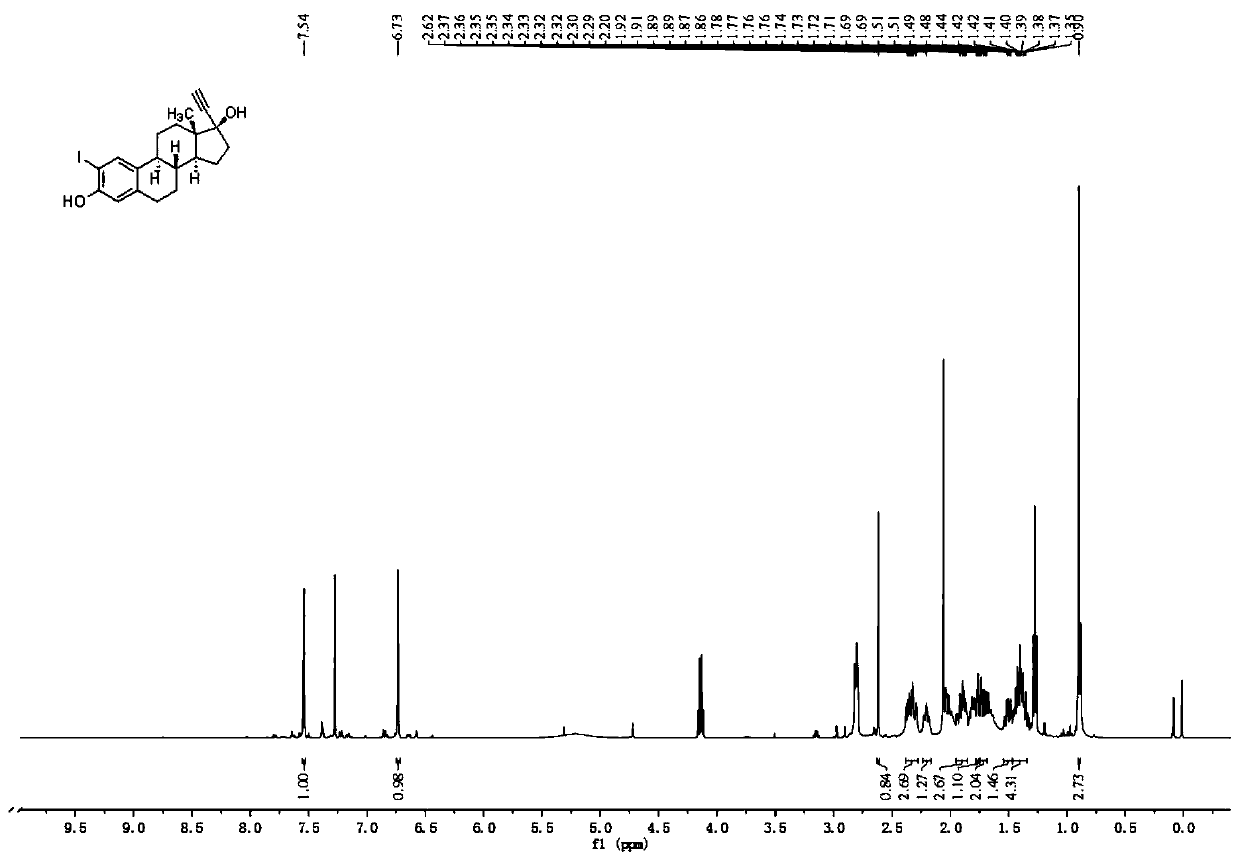
[0039] Embodiment 1: Preparation of 2-iodoethinyl estradiol intermediate
[0040]
[0041]Add 0.2 mmol of ethinyl estradiol (EE) to a 25 mL round bottom flask, then add 5 mL of acetonitrile solvent to dissolve, add 0.01 mmol (1 equiv) of indium trifluoromethanesulfonate, and 0.11 mmol of N-iodosuccinimide mmol (11 equiv), the reaction was stirred at room temperature for 8-10 h, and the reaction process was monitored by a TLC plate. After the reaction is over, add about 15 mL of saturated aqueous sodium chloride solution to the reaction solution, extract with dichloromethane, take the organic layer, dry it over anhydrous sodium sulfate, filter, and remove the organic solvent under reduced pressure with a rotary evaporator to obtain monoiodo Crude EEI compound. The crude product of the EEI compound was subjected to silica gel column chromatography, using a solution with a volume ratio of ethyl acetate and petroleum ether of 1:3 as the mobile phase, followed by TLC to collect...
Embodiment 2
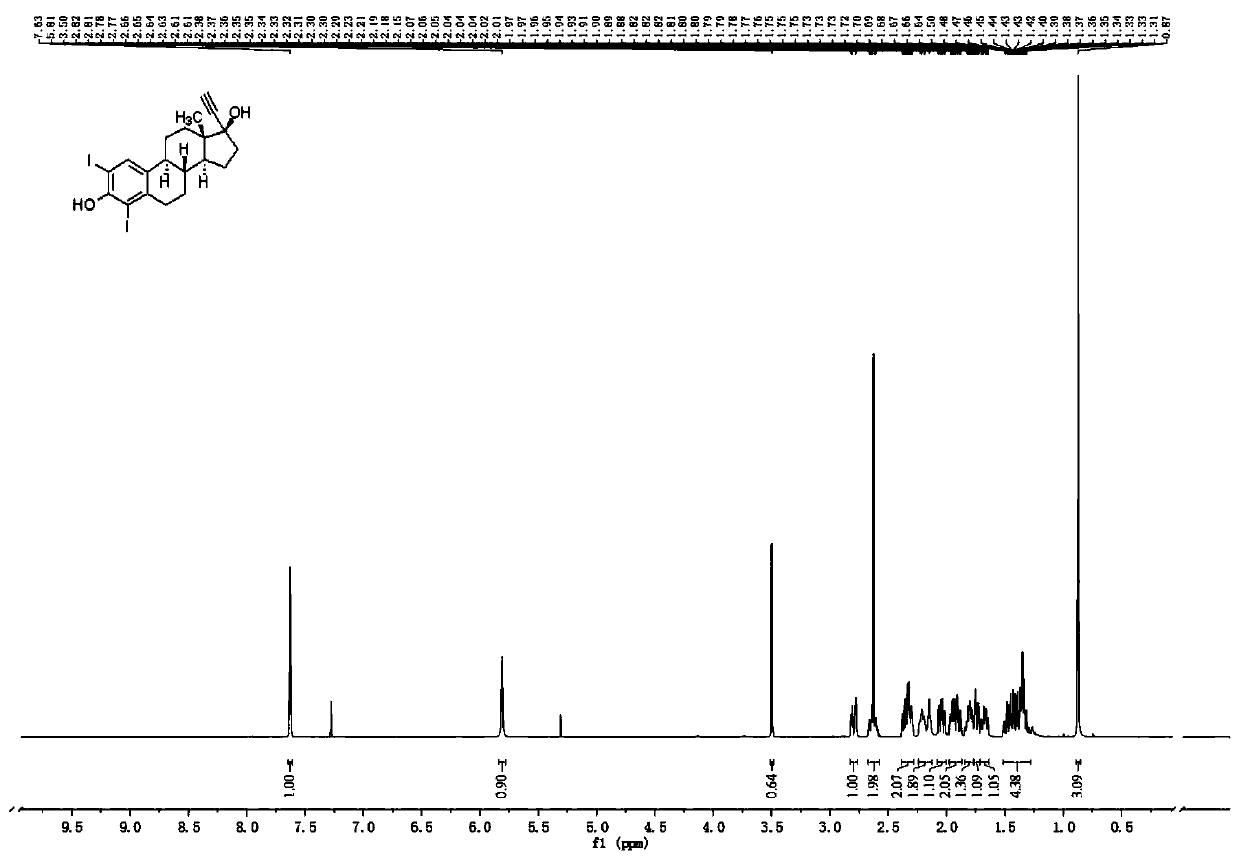
[0043] Embodiment 2: Preparation 2,4-diiodoethinyl estradiol intermediate
[0044]
[0045] Add ethinyl estradiol (EE) 0.3mmol in the 25mL round-bottomed flask, then add 10mL of absolute ethanol to dissolve, add iodine simple substance 0.15mmol (0.5equiv), 30% hydrogen peroxide 0.6mmol (2equiv), 50 The reaction was stirred at ℃ for 10-12 h, and the reaction process was monitored with a TLC plate. After the reaction was completed, 20 mL of saturated aqueous sodium chloride solution was added to the reaction liquid, extracted with dichloromethane, the organic layer was dried over anhydrous sodium sulfate, filtered, and the organic solvent was removed by a rotary evaporator under reduced pressure to obtain a crude product of EII compound. Put the EII compound crude product into silica gel column chromatography, use ethyl acetate and petroleum ether as the mobile phase with a volume ratio of 1:3, TLC tracking and collecting the eluent with an Rf value of 0.3-0.6, and collect th...
Embodiment 3
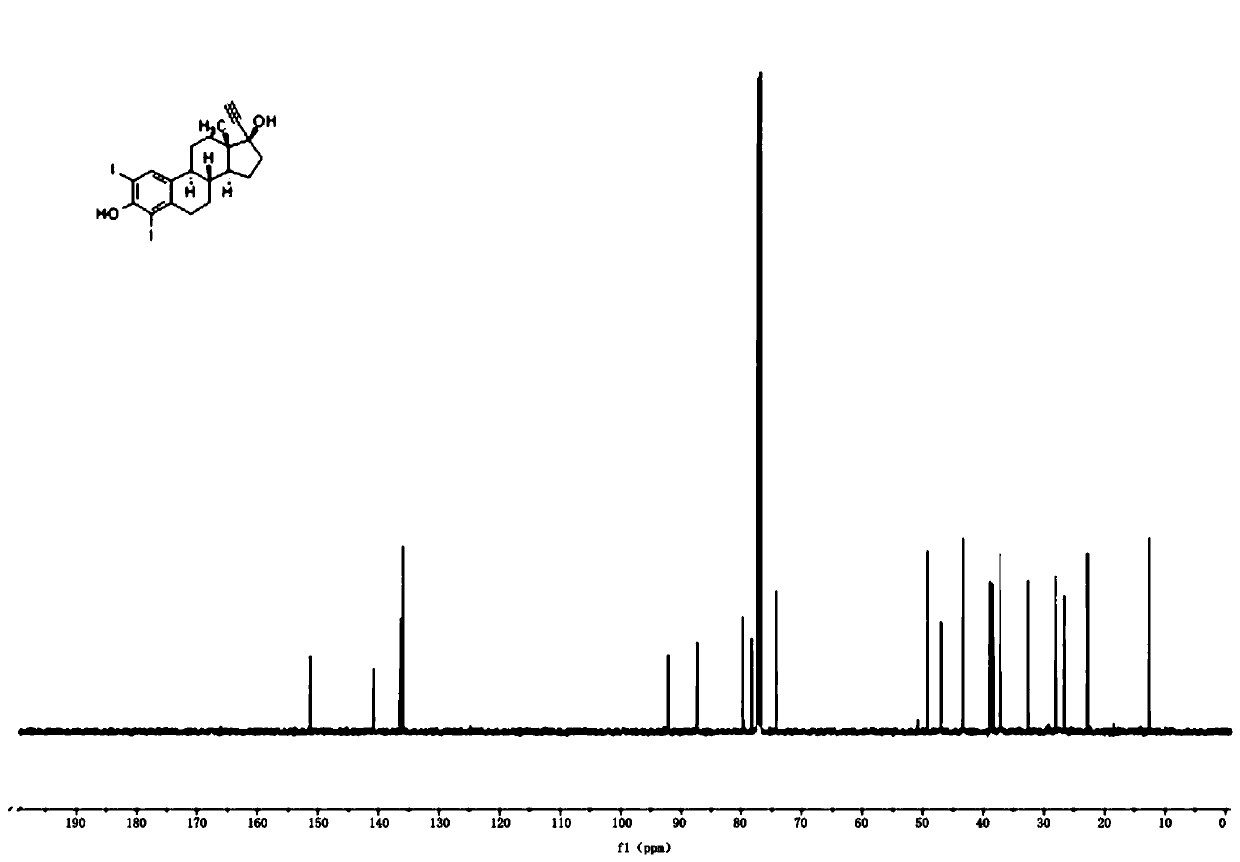
[0047] Example 3: Preparation of ethinyl estradiol modified drug EEX-1
[0048]
[0049] Weigh compound (Example 1) EEI 0.4mmol and add 10mL of N,N-dimethylformamide solvent, add 0.48mmol (1.2equiv) ethyl acrylate, 0.04mmol (0.1equiv) palladium acetate, 0.8mmol (2equiv) triethylamine, under anhydrous and oxygen-free conditions, stirred and reacted at 90°C for 4-8h at high temperature. After the reaction, 30mL of saturated aqueous sodium chloride solution was added to the reaction solution, and extracted three times with dichloromethane, and the organic The phase was dried with anhydrous sodium sulfate, filtered, rotary evaporated under reduced pressure, and vacuumized to obtain the crude product of EEX-1 compound. The crude product of the EEX-1 compound was subjected to silica gel column chromatography, using a solution with a volume ratio of ethyl acetate and petroleum ether of 1:12 as the mobile phase, and followed by TLC to collect the eluent with an Rf value of 0.4-0.5,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com