Divalent europium activated lithium borate scintillation glass and preparation method thereof
A technology of scintillation glass and lithium borate, applied in glass manufacturing equipment, glass molding, manufacturing tools, etc., can solve the problems of increasing the complexity of scintillation glass preparation, unfavorable industrial production, limiting capture capacity, etc. The effect of detection efficiency, improved capture cross section, and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0027] Taking Example 1 as an example to illustrate the preparation process of the scintillation glass.
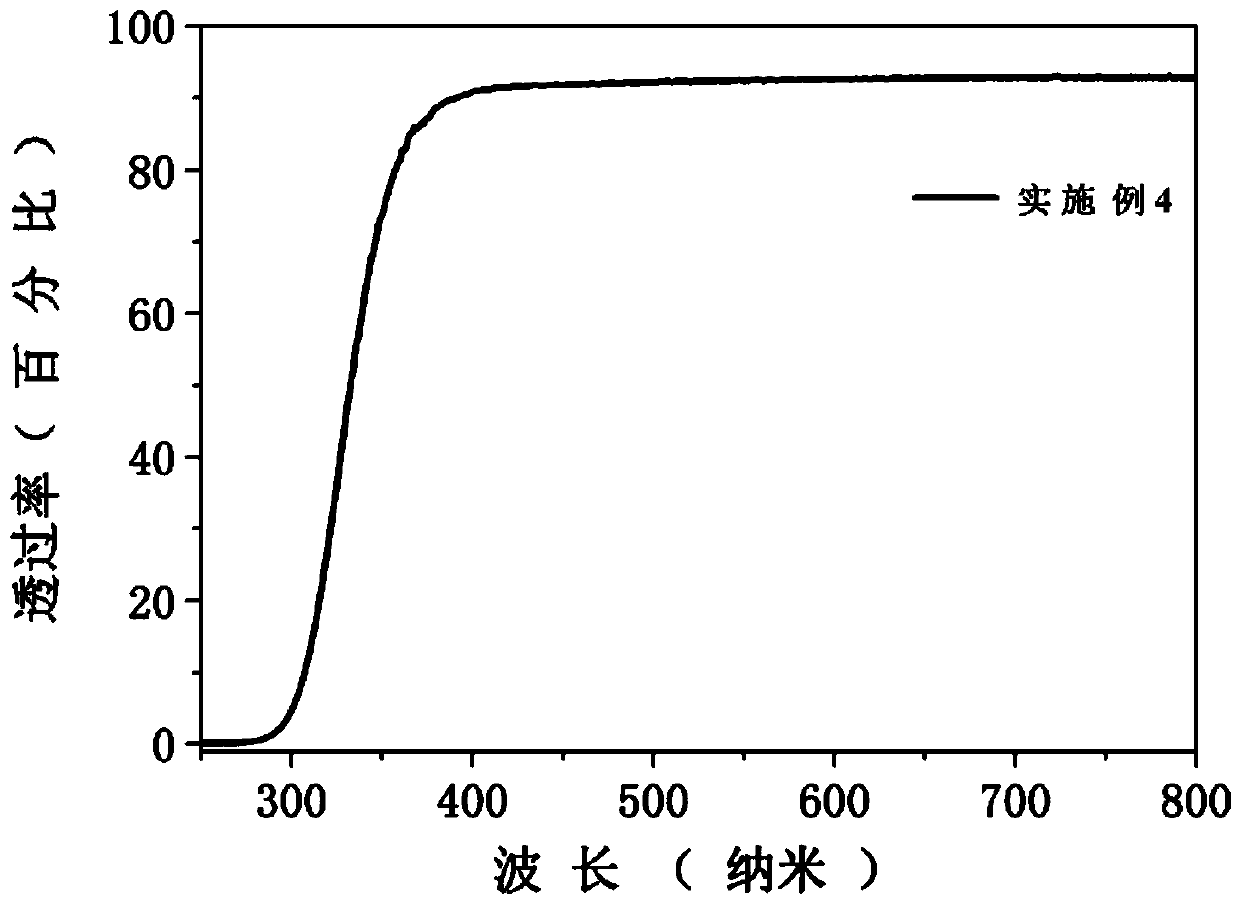
[0028] Accurately weigh the glass raw material according to the specific glass composition of Example 1 in Table 1, and fully grind the glass raw material in an agate mortar for 15 minutes, then directly put it into a high-temperature electric furnace at 1050 ° C and keep it warm for 50 minutes in an air atmosphere to obtain a uniform melt . Then pour the above homogeneous melt into a stainless steel mold with a preheating temperature of 450°C for casting, and quickly place the formed glass in a muffle furnace at 450°C for 3 hours for annealing treatment. Cut into 10×10×2mm 3 The scintillation glass of the present invention is obtained after regular shape, surface grinding and polishing.
[0029] Examples 2-6 The procedure for preparing glass is the same as that of Example 1, except for the melting temperature and annealing time. With Li in the scintillation glass 2 Th...
Embodiment 7-11
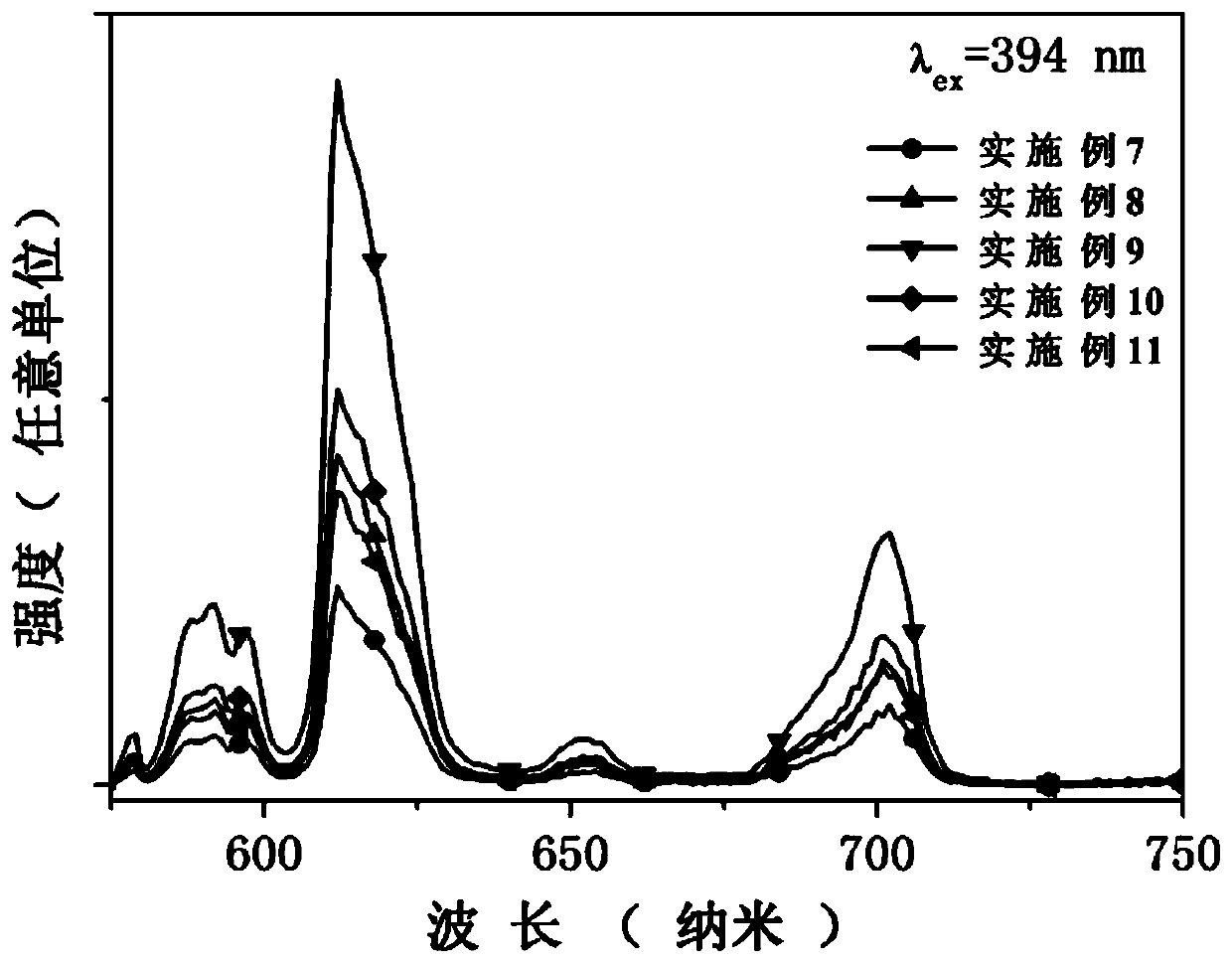
[0034] The preparation process of all scintillation glasses in Examples 7-11 in Table 2 is the same.
[0035] According to the specific glass composition of Examples 7-11 shown in Table 2, the glass raw materials are accurately weighed, and after the glass raw materials are fully ground in an agate mortar for 15 minutes, they are directly placed in a 990°C high-temperature electric furnace and kept in an air atmosphere for 40 minutes to obtain homogeneous melt. Then pour the above homogeneous melt into a stainless steel mold with a preheating temperature of 380°C for casting, and quickly place the formed glass in a muffle furnace at 380°C for 3 hours for annealing treatment. Cut into 10×10×2mm 3 The scintillation glass of the present invention is obtained after regular shape, surface grinding and polishing.
[0036] Table 2. Embodiment 7-11 glass composition (mol%)
[0037] Example Li 2 o
[0038] Use the fluorescence spectrometer (Edinburgh instrument company...
Embodiment 12-16
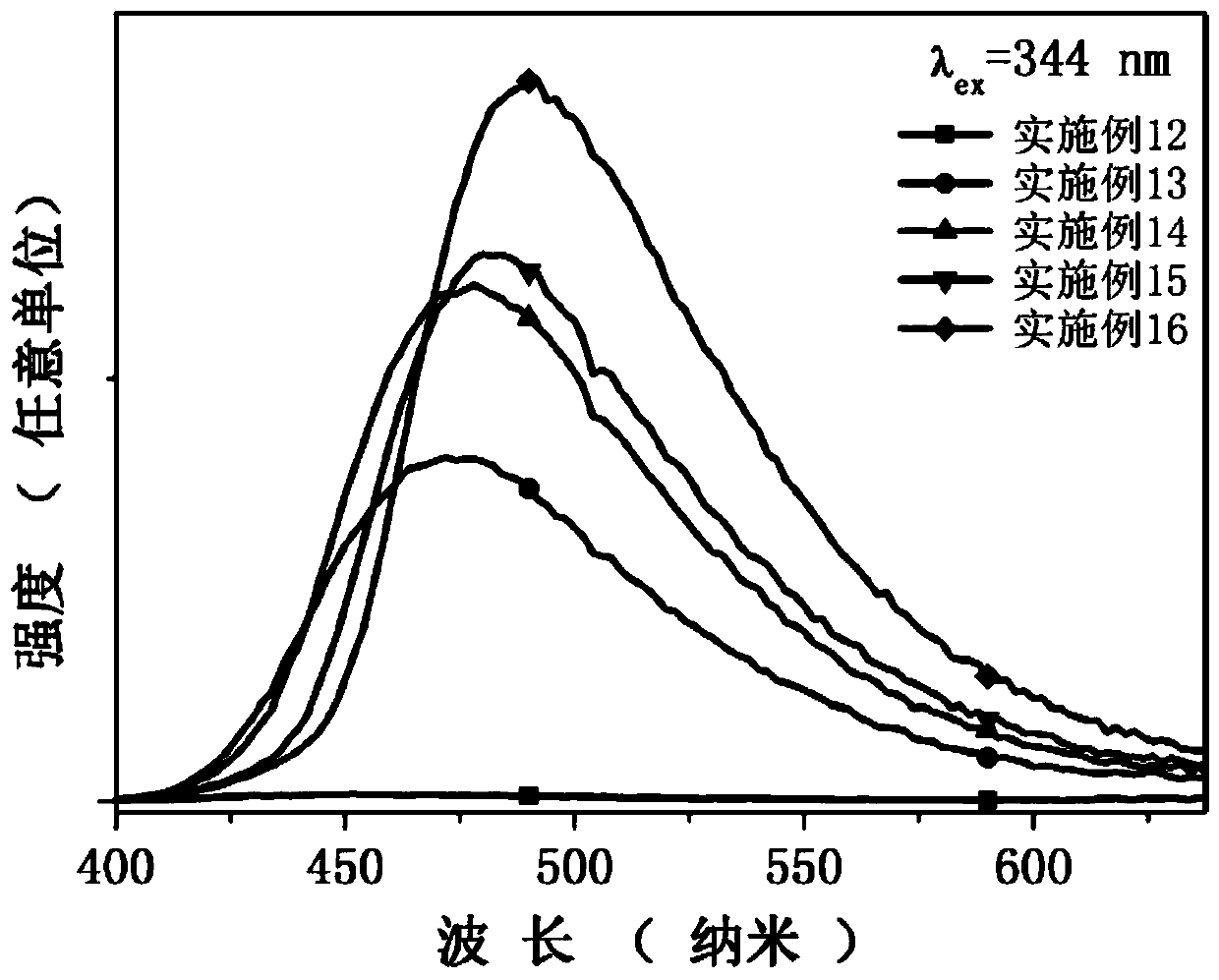
[0040] All scintillation glasses of Examples 12-16 in Table 3 (actually, Example 12 has the same components as Example 8) have the same preparation process.
[0041] According to the specific glass composition of Examples 12-16 shown in Table 3, the glass raw materials are accurately weighed, and after fully grinding the glass raw materials in an agate mortar for 15 minutes, they are directly placed in a high-temperature electric furnace at 1000 ° C and kept warm for 40 minutes in an air atmosphere to obtain homogeneous melt. Then pour the above homogeneous melt into a stainless steel mold with a preheating temperature of 410°C for casting, and quickly place the formed glass in a muffle furnace at 410°C for 3 hours for annealing treatment. Cut into 10×10×2mm 3 The scintillation glass of the present invention is obtained after regular shape, surface grinding and polishing.
[0042] Table 3. Embodiment 12-16 glass composition (mol%)
[0043] Example Li 2 o
B ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com