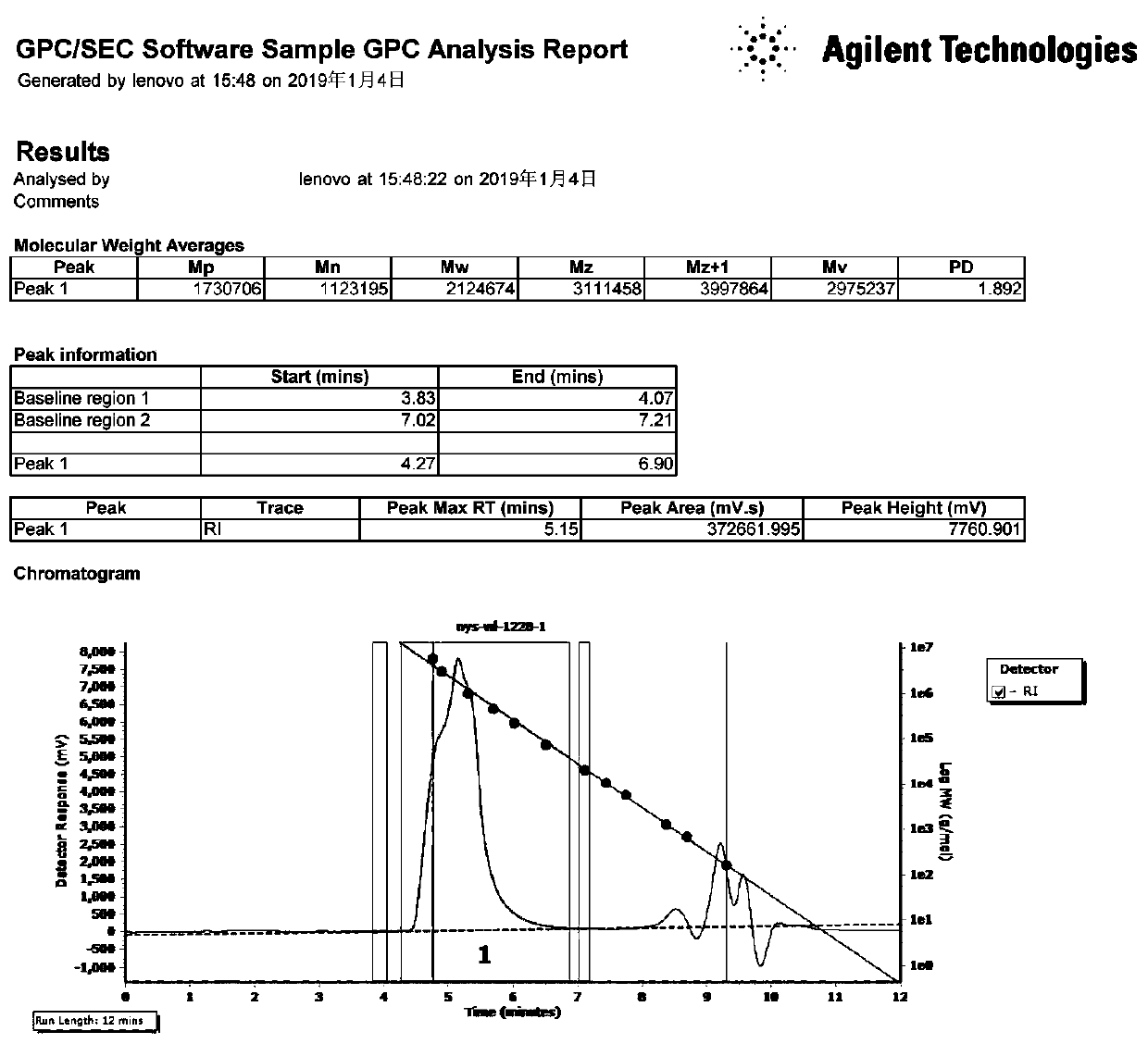
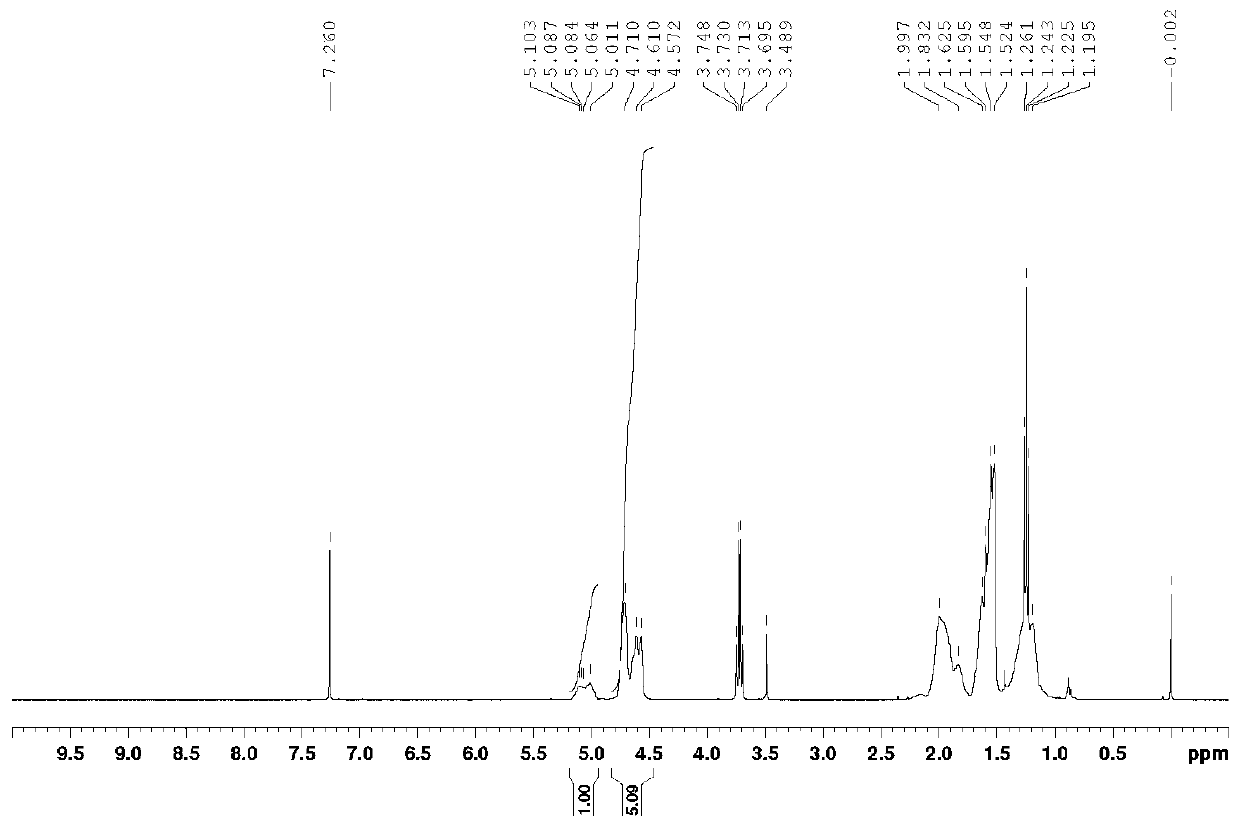
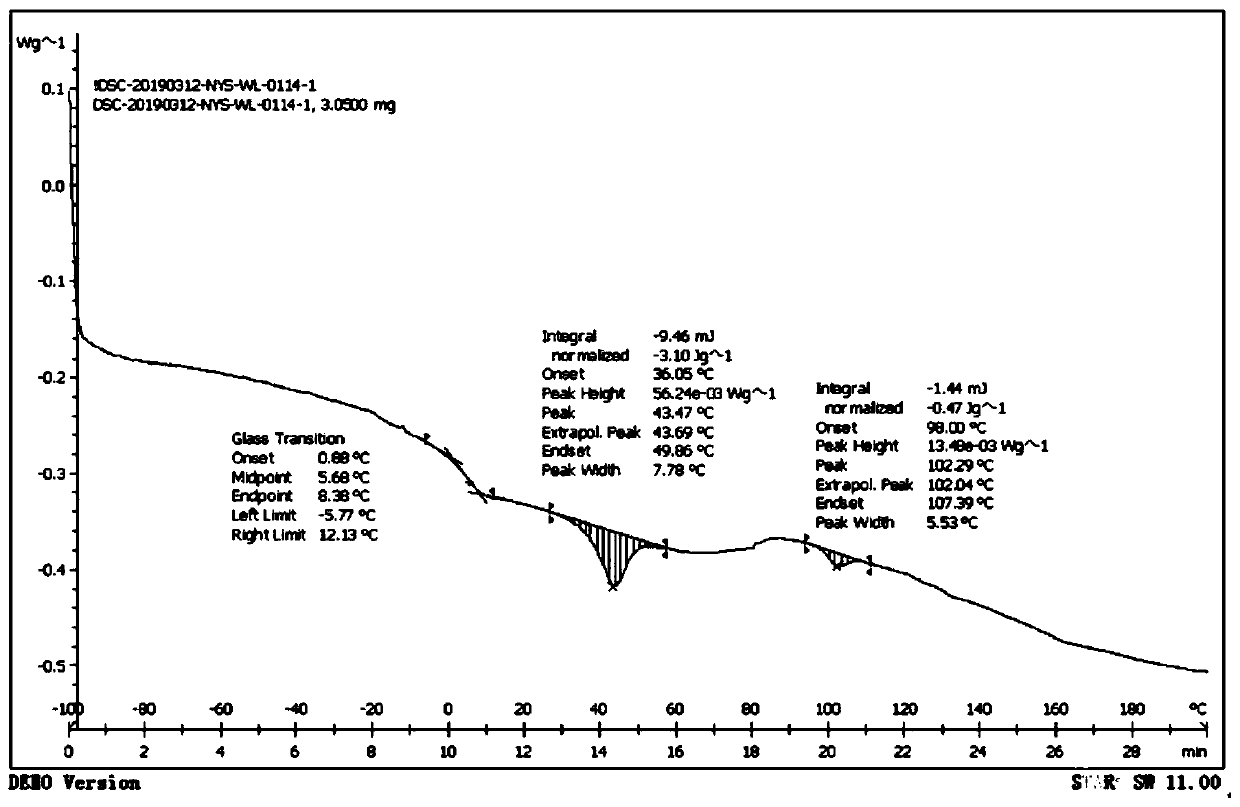
Bipyridine iron complex, preparation method thereof and application of complex in conjugated diene polymerization
A technology of bipyridyl iron and conjugated diene, which is applied in the direction of iron organic compounds, iron group organic compounds without C-metal bonds, etc., can solve the problems of lack of efficient preparation, etc., and achieve simple and easy preparation, good solubility, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1. Preparation of bipyridyl iron complex catalyst 1 (structural formula is as follows).
[0043]
[0044] Add anhydrous Fe(acac) to a 50 mL Schlenk bottle under argon atmosphere 2 (127.0mg, 0.5mmol), dissolved in 6mL of absolute ethanol at 60°C; then a solution of 2,2'-bipyridine (78.0mg, 0.5mmol) in ethanol (4mL) was added dropwise to the system. React at 60°C for half an hour, then return to room temperature and stir overnight. The filtrate was collected by filtration, concentrated, washed twice with cold ethanol, and vacuum-dried for 12 h to obtain product 1 as a brown-yellow solid with a yield of 68%.
[0045] Mass Spectrometry: C 20 h 22 FeN 2 o 4 :[M+H]+: theoretical value: 411.1002; measured value: 410.0998.
[0046] Elemental Analysis: C 20 h 22 FeN 2 o 4 : Theoretical value: C, 58.55%; H, 5.41%; N, 6.83%; Measured value: C, 58.34%; H, 5.53%; N, 7.09%.
Embodiment 2
[0047] Example 2. Preparation of pyridine imine iron complex catalyst 2 (structural formula is as follows).
[0048]
[0049] Add anhydrous Fe(acac) to a 50 mL Schlenk bottle under argon atmosphere 3 (211.8mg, 0.6mmol), dissolved in 6mL of absolute ethanol at 60°C; then a solution of 2,2'-bipyridine (93.6mg, 0.6mmol) in ethanol (4mL) was added dropwise to the system. React at 60°C for half an hour, then return to room temperature and stir overnight. The filtrate was collected by filtration, concentrated, washed twice with cold ethanol, and dried in vacuum for 12 h to obtain product 2 as a reddish-brown solid with a yield of 78%.
[0050] Mass Spectrometry: C 25 h 29 FeN 2 o 6 :[M+H]+: theoretical value: 510.1448; measured value: 510.1443.
[0051] Elemental Analysis: C 25 h 29 FeN 2 o 6 : Theoretical value: C, 58.95%; H, 5.74%; N, 5.50%; Measured value: C, 58.54%; H, 5.61%; N, 5.85%.
Embodiment 3
[0052] Example 3. Preparation of pyridine imine iron complex catalyst 3 (structural formula is as follows).
[0053]
[0054] Add anhydrous Fe(acac) to a 50 mL Schlenk bottle under argon atmosphere 2 (127mg, 0.5mmol), dissolved in 6mL of absolute ethanol at 60°C; then, a solution of 5,5'-dimethyl-2,2'-bipyridine (92.0mg, 0.5mmol) in ethanol (4mL) was dropped added to the system. React at 60°C for half an hour, then return to room temperature and stir overnight. The filtrate was collected by filtration, concentrated, washed twice with cold ethanol, and dried in vacuum for 12 h to obtain tan solid product 3 with a yield of 55%.
[0055] Mass Spectrometry: C 22 h 26 FeN 2 o 4 :[M+H]+: theoretical value: 439.1315; measured value: 439.1319.
[0056] Elemental Analysis: C 22 h 26 FeN 2 o 4 : Theoretical value: C, 60.29%; H, 5.98%; N, 6.39%; Measured value: C, 59.90%; H, 6.21%; N, 6.65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com