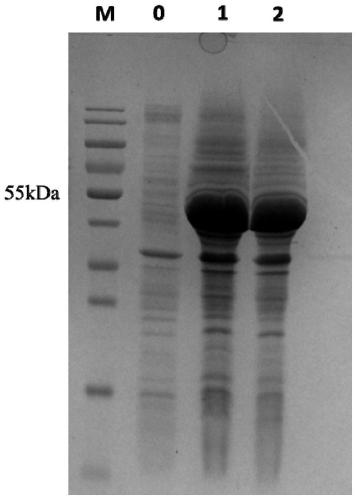
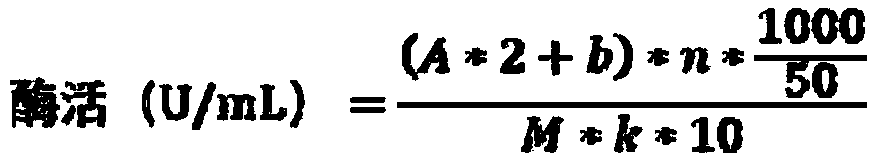Escherichia coli for producing sucrose phosphorylase
A technology of Escherichia coli and glucose phosphate, applied in the field of microorganisms, can solve the problems of low expression, difficult to meet the requirements of industrial applications, and low enzyme activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The construction of embodiment 1 Escherichia coli SPL02 genetically engineered bacteria
[0024] (1) Gene optimization: Sucrosephosphorylase (GenBank Accession NO.D90314) derived from Leuconostoc mesenteroides ATCC 12291 was analyzed and optimized to obtain the gene sequence shown in SEQ ID NO.1, and two restriction sites NcoI and XhoI were added to The two ends of the optimized sucrose phosphorylase are synthesized to obtain the SPase gene.
[0025] (2) Construction of genetically engineered bacteria: the synthetic SPase gene and pET-28a vector were double-digested with restriction endonucleases NcoI and XhoI, and the products after digestion were connected with Solution I, and then the recombinant vector pET-28a-SPase Transformed into Escherichia coli BL21 (DE3) for expression.
Embodiment 2
[0026] The preparation of embodiment 2 recombinant sucrose phosphorylase
[0027] Inoculate a single colony of the recombinant strain in the LB liquid medium containing Kana, cultivate overnight at 37°C, 200r / min, and insert it into the TB liquid medium containing Kana at 37°C, 200r / min Min culture to bacterial density OD 600 After reaching 0.6, add IPTG inducer and induce at 25°C, 200r / min for 24h, then collect the bacterial liquid. Centrifuge the bacterial liquid in a low-temperature refrigerated centrifuge at 7000 r / min at 4°C for 15 minutes to collect the bacterial cells. Bacteria use 50mmol / L K 2 HPO4 / KH 2 Bacteria were collected after washing twice with PO4 buffer (pH6.5). Add the collected wet bacteria to 50mmol / L K 2 HPO4 / KH 2 PO4 buffer (pH 6.5) buffer solution was used to make a bacterial suspension, placed on ice and fixed, and then the bacterial cells were disrupted by ultrasonic waves. Ultrasonic breaker working time: 2s, intermittent time: 4s, total time: ...
Embodiment 3
[0029] The enzyme activity assay of embodiment 3 recombinant sucrose phosphorylase
[0030]In phosphate buffer, sucrose phosphorylase can catalyze sucrose and inorganic phosphoric acid to generate glucose-1-phosphate and D-fructose. The sucrose hydrolysis reaction can be carried out first, and then the content of the generated D-fructose can be detected by DNS to determine the sucrose phosphate [Choi H.C.,Seo D.H.,Jung J.H.,et al.Developmemt of new assay for sucrosephosphorylase and its application to the characterization of Bifidobacterium longumSJ32 sucrosephosphorylase[J].Food Science and Biotechnology,2011,20(2):513-51 .
[0031] The enzyme activity determination method refers to [Wu Jing, Wu Dan, Zhu Jie, et al. A recombinant Bacillus subtilis expressing sucrose phosphorylase derived from L. mesenteroides. Chinese invention patent application, application number: 201710637427.6, publication number: CN107236696A]. The assay steps include: 500 μL of 5% sucrose solution, 50...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific enzyme activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com