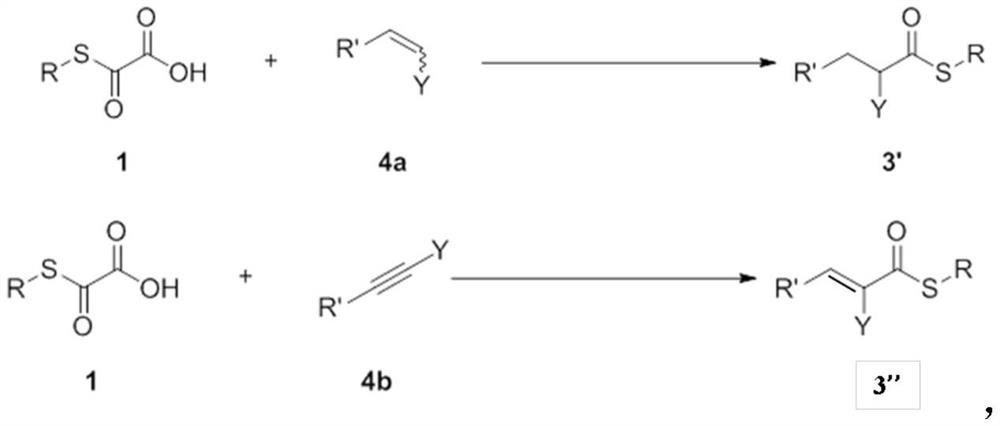
Process for preparing thioesters from oxothioglycolic acid compounds
A technology for thioglycolic acid and compounds, which is applied in the field of palladium-catalyzed preparation of thioesters by oxothioglycolic acid compounds, can solve problems such as complicated operations, and achieve the effects of simple operations, simplified processing methods, and optimized synthesis strategies.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1, preparation S-(p-tolyl) 4-acetophenone thioester
[0066]
[0067] Following general procedure A, the yields are shown in Table 1.
[0068] Utilize nuclear magnetic resonance to analyze the S-(p-tolyl) 4-acetophenone thioester obtained in embodiment 1, obtain result:
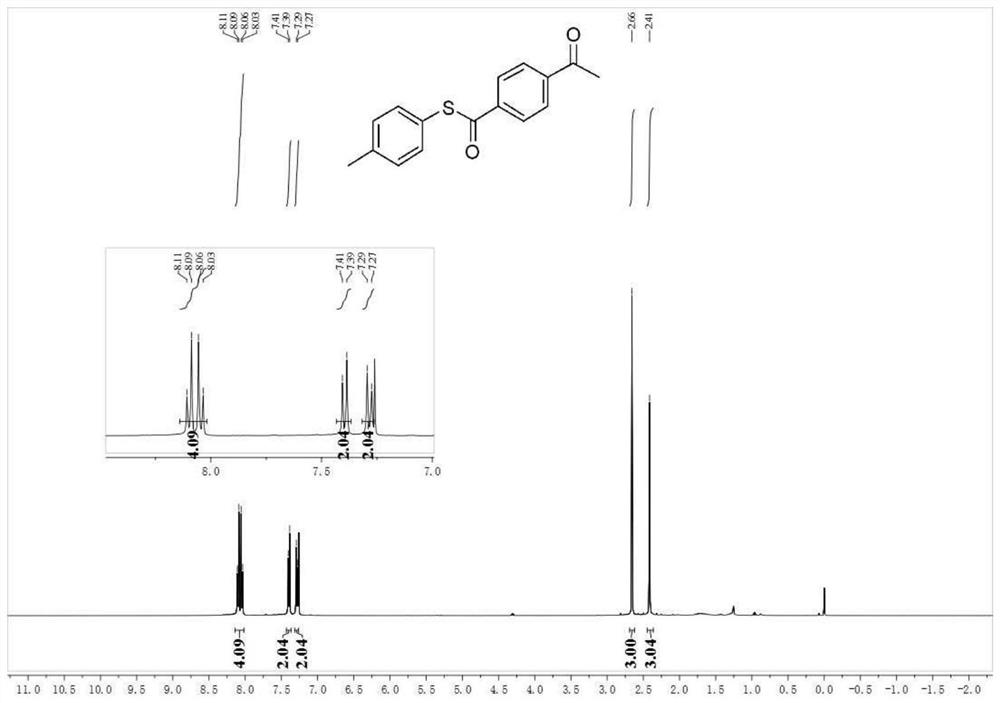
[0069] 1 H NMR (400MHz, CDCl 3 )δ8.07(dd, J=20.9,8.5Hz,4H),7.40(d,J=8.1Hz,2H),7.28(d,J=7.9Hz,2H),2.66(s,3H),2.41( s,3H). See figure 1 .
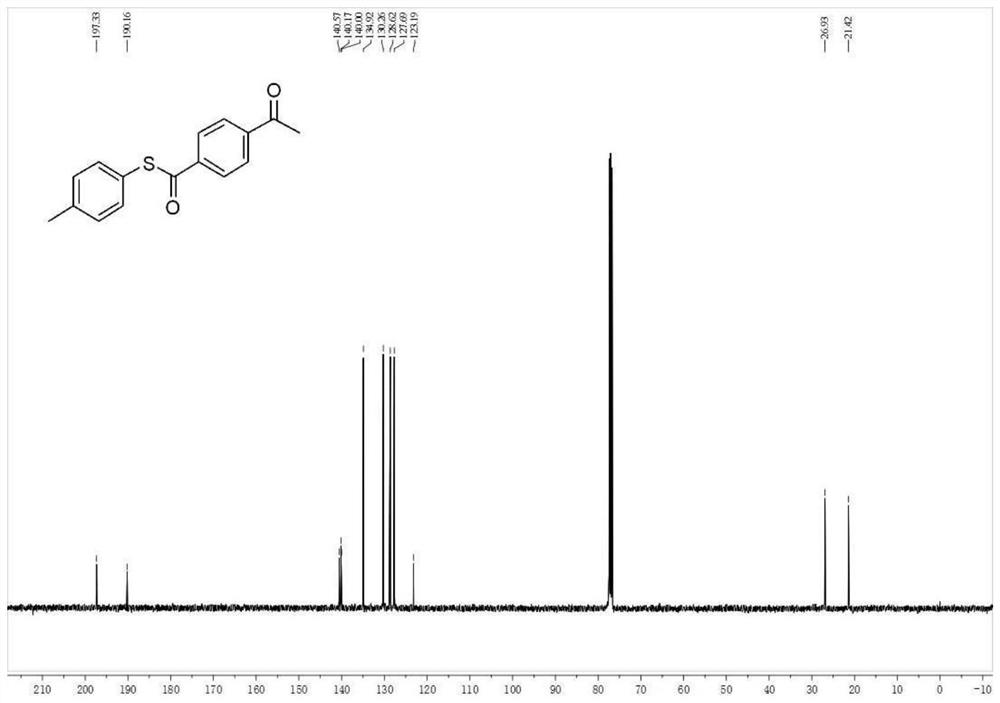
[0070] 13 C NMR (101MHz, CDCl 3)δ 197.3, 190.2, 140.6, 140.2, 140.0, 134.9, 130.3, 128.6, 127.7, 123.2, 26.9, 21.4. See figure 2 .
Embodiment 2
[0071] Embodiment 2, preparation S-(p-tolyl) 4-formylbenzenethio ester
[0072]
[0073] Following general procedure A, the yields are shown in Table 1.
[0074] Utilize nuclear magnetic resonance to analyze the S-(p-tolyl) 4-formylphenyl thioester obtained in embodiment 2, obtain result:
[0075] 1 H NMR (400MHz, CDCl 3 )δ10.10(s,1H),8.16(d,J=8.0Hz,2H),7.99(d,J=8.1Hz,2H),7.39(d,J=7.9Hz,2H),7.34–7.21( m,2H),2.41(s,3H).
[0076] 13 C NMR (101MHz, CDCl 3 )δ191.4, 190.1, 141.1, 140.3, 139.5, 134.9, 130.3, 129.9, 128.0, 123.1, 21.4.
Embodiment 3
[0077] Embodiment 3, preparation S-(p-tolyl) 4-fluorophenylthio ester
[0078]
[0079] Following general procedure A, the yields are shown in Table 1.
[0080] Utilize nuclear magnetic resonance to analyze the S-(p-tolyl) 4-fluorophenylthio ester obtained in embodiment 3, obtain the result:
[0081] 1 H NMR (400MHz, CDCl 3 )δ8.10–8.00(m,2H),7.42–7.35(m,2H),7.27(d,J=7.9Hz,2H),7.21–7.10(m,2H),2.41(s,3H).
[0082] 13 C NMR (101MHz, CDCl 3 )δ189.2, 166.05(d, J=255.3Hz), 140.0, 135.1, 133.03(d, J=3.1Hz), 130.2, 130.06(d, J=9.3Hz), 123.5, 115.90(d, J=22.1Hz) ,21.4.
[0083] 19 F NMR (376MHz, CDCl 3 )δ-105.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com