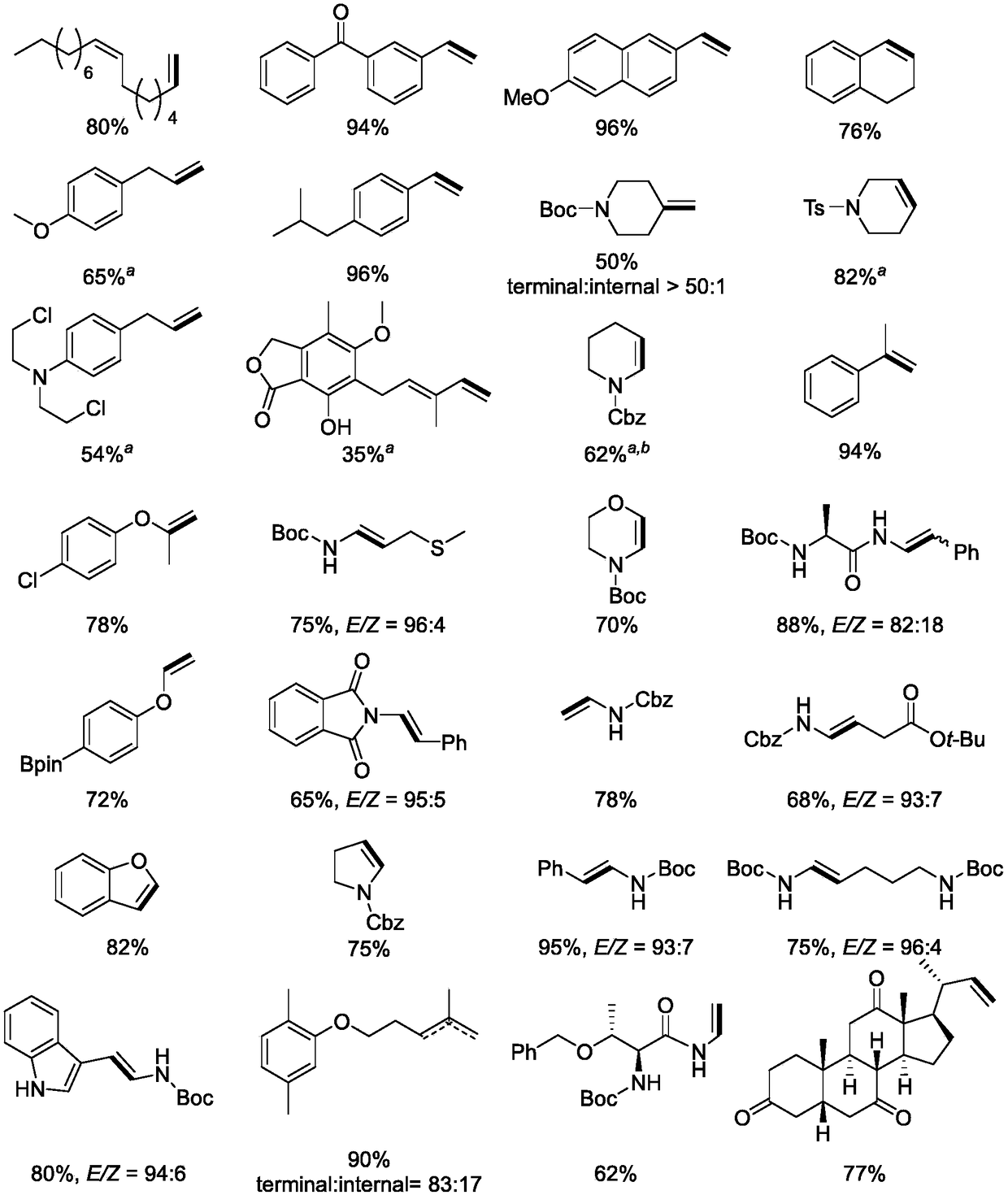
Preparation method of olefin and method for synthesizing Chondriamide A and Chondriamide C
An olefin and hydrocarbon-based technology, applied in the preparation of organic compounds, the preparation of amino compounds from amines, the preparation of carbon-based compounds, etc., can solve the problems of high reaction temperature and environmental pollution, and achieve the advantages of simple operation, cost reduction, and simplified treatment methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The invention provides a kind of preparation method of olefin, comprising:
[0044] Mixing a compound of formula (I), a palladium catalyst, a phosphorus ligand, a base, and an organic solvent for light reaction at room temperature to obtain an alkene having a structure of formula (II);
[0045]
[0046] Among them, R 1 C1-C50 alkyl group, C1-C50 heteroatom-containing alkyl group, C2-C50 unsaturated hydrocarbon group, C2-C50 heteroatom-containing unsaturated hydrocarbon group or C3-C50 aromatic group;
[0047] X is a hydrogen atom, methylene, oxygen or nitrogen;
[0048] When X is a hydrogen atom, R 2 group does not exist;
[0049] When X is methylene, oxygen or nitrogen, R 2 C1-C50 alkyl group, C1-C50 heteroatom-containing alkyl group, C2-C50 unsaturated hydrocarbon group, C2-C50 heteroatom-containing unsaturated hydrocarbon group, C3-C50 aromatic group or X and R 2 Form a five- or six-membered ring;
[0050] or R 1 , X and their carbons form a ring;
[0051...
Embodiment 1
[0078] Embodiment 1, preparation 1-pentadecene
[0079] Reaction formula:
[0080]
[0081] The specific method is as follows:
[0082] Add photocatalyst palladium chloride (2mol%, 0.7mg), 4,5-bis-diphenyl Phosphine-9,9-dimethylxanthene (L1) (3mol%, 3.5mg), 2-(dicyclohexylphosphino)biphenyl (L2) (4mol%, 2.8mg) and active aliphatic NHPI Ester (0.2 mmol, 80.2 mg). The air in the tube was completely replaced with argon three times, and then 2,4,6-collidine (0.2 mmol, 24.2 mg), 2 mL of N,N-dimethylacetamide (DMA) were added under argon atmosphere. The reaction system was continuously stirred at room temperature for 15 hours under the irradiation of a 36W blue LED lamp (IKA magnetic stirrer, RCT basic type, stirring speed 500 rpm). After the reaction is complete, use H 2The reaction was quenched with O, and the reaction solution was extracted with ethyl acetate (3*10 mL), and then the combined organic phase was concentrated by rotary evaporation (BUCHI Co., Ltd., BUCHI rota...
Embodiment 2
[0086] Embodiment 2, preparation Z-octadeca-1,8-diene
[0087] Reaction formula:
[0088]
[0089] Method is with example 1, and productive rate sees figure 1 .
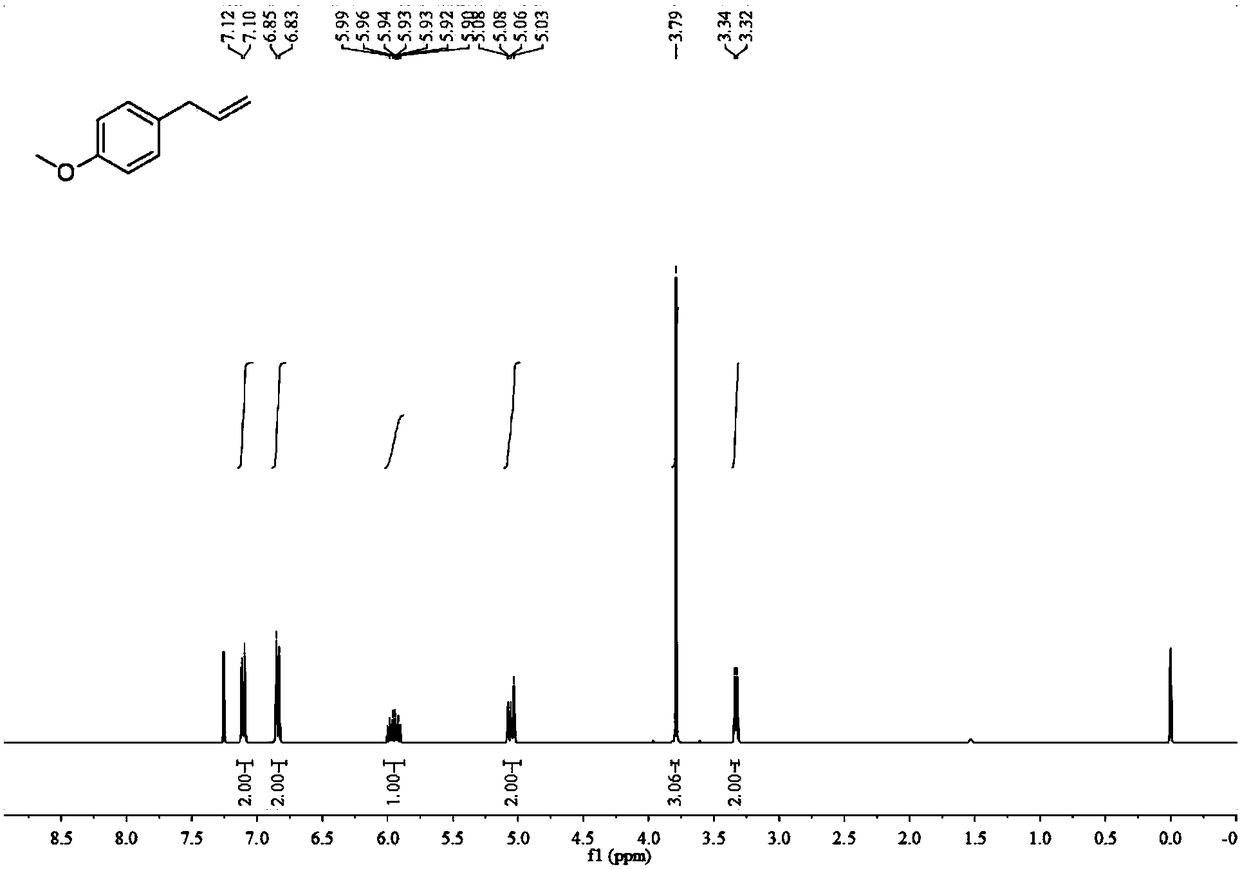
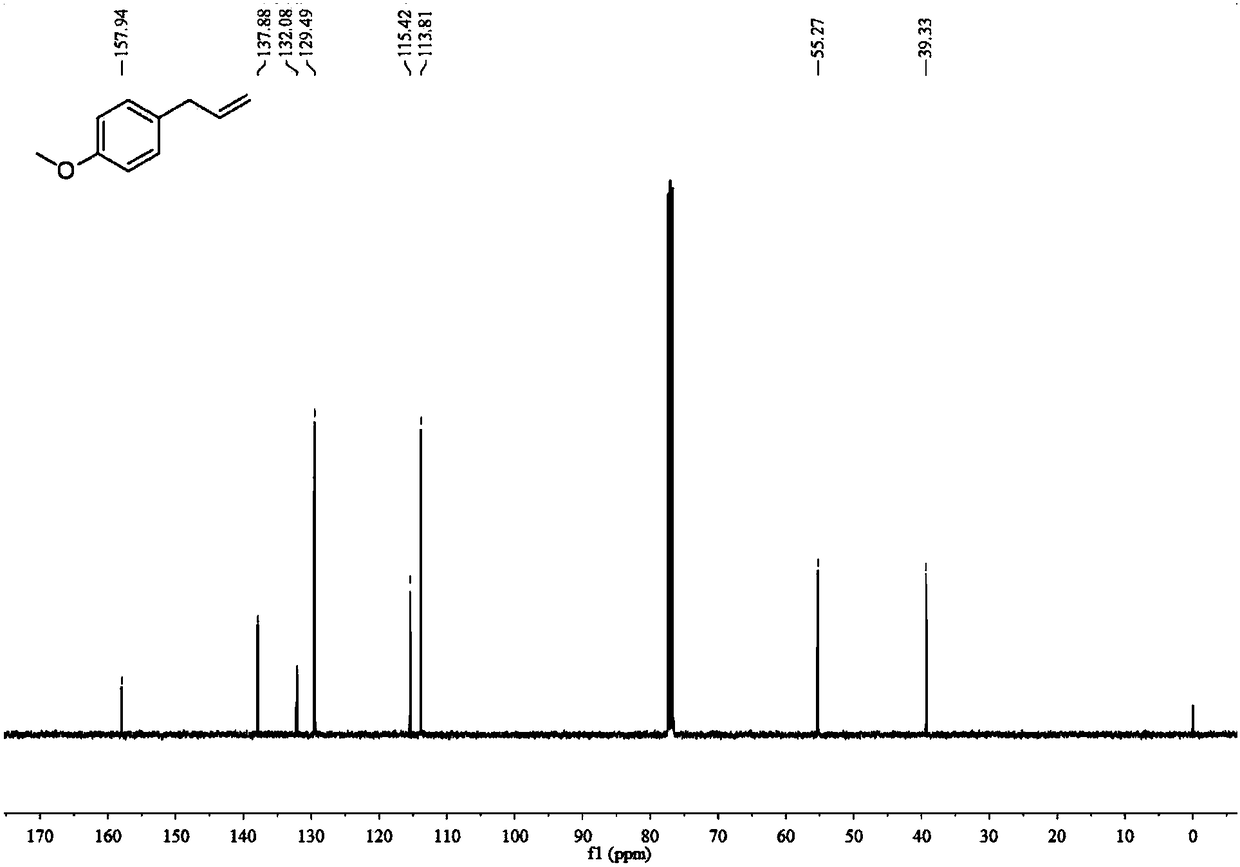
[0090] Utilize nuclear magnetic resonance to analyze the Z-octadeca-1,8-diene obtained in embodiment 2, obtain the result:
[0091] 1 H NMR (400MHz, CD2Cl2) δ5.81 (ddt, J=16.9, 10.2, 6.7Hz, 1H), 5.38-5.33(m, 2H), 5.04-4.90(m, 2H), 2.08-1.98(m, 6H ), 1.41-1.25(m, 18H), 0.88(t, J=6.9Hz, 3H).
[0092] 13 C NMR (101MHz, CD2C12) δ139.2, 130.0, 129.8, 114.2, 33.8, 31.9, 29.8, 29.6, 29.5, 29.3, 28.9, 28.8, 27.2, 27.1, 22.7, 14.1.(one carbon signal is overlapped)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com