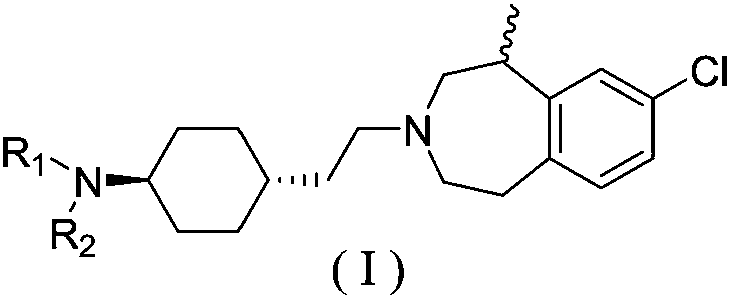
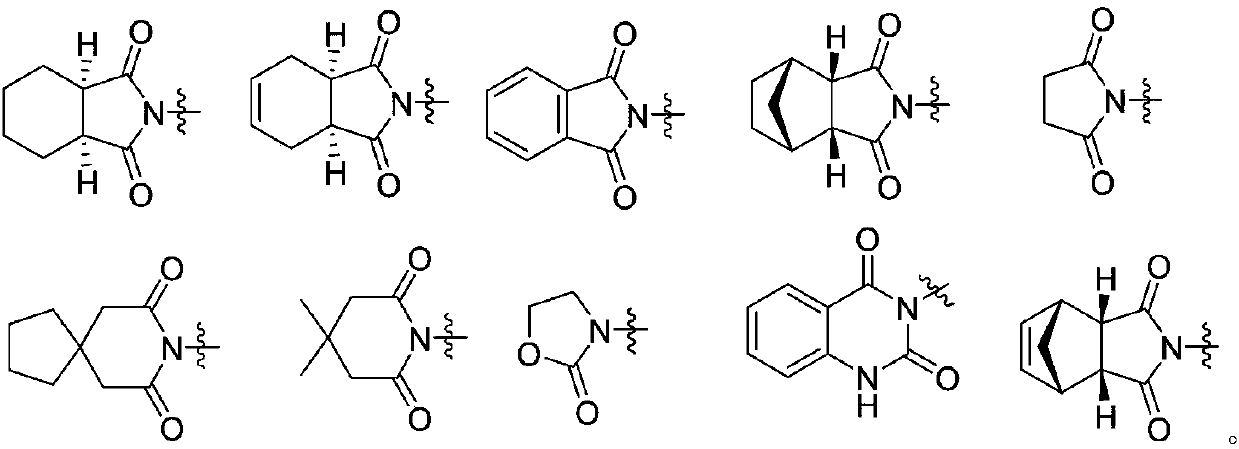
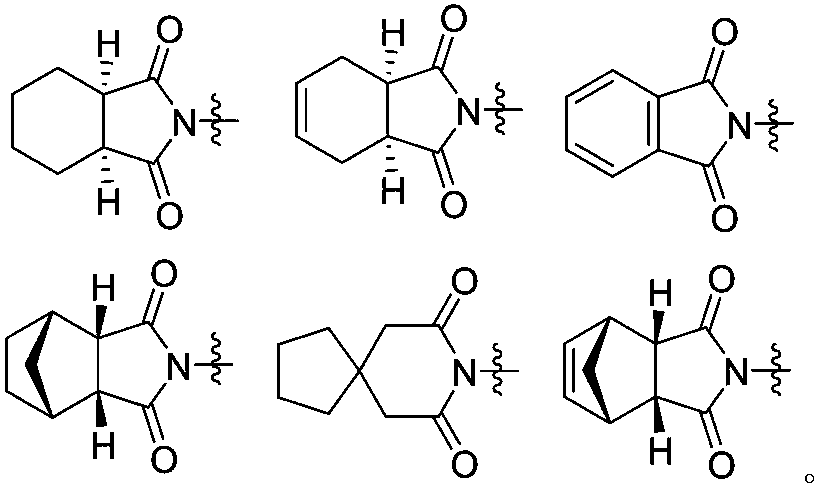
Heptazine type compound and application thereof in preparing anti-schizophrenia medicine
A compound and heptazine technology, applied in the application field of anti-schizophrenia drugs, can solve the problems of interfering with the objective evaluation of animal cognitive function behavioral results, difficult for patients to return to society, and reduced medication compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] N-(trans-4-(2-((R)-8-chloro-1-methyl-1,2,4,5-tetrahydro-3H-benzo[d]azepine-3-yl ) ethyl) cyclohexyl) acetamide (I-1) and the preparation of salts thereof
[0122] Trans-4-(2-((R)-8-chloro-1-methyl-1,2,4,5-tetrahydro-3H-benzo[d]azepine-3-yl)ethyl Base) cyclohexyl-1-amine (6-1, prepared according to general method 1) (1.0g, 3.1mmol), triethylamine (4.7mmol) were added to CH 2 Cl 2 (20mL), stirred, 0 ~ 5 ℃ dropwise added acetyl chloride (0.3g, 3.4mmol) CH 2 Cl 2 (5mL) solution, dropwise, stirred at room temperature for 2-4h, the system was washed successively with water and saturated brine, the organic layer was concentrated to dryness, and recrystallized with absolute ethanol to obtain 0.9g of white solid, with a yield of 78%.
[0123] 1 H NMR (Chloroform-d, δ:ppm) δ7.15(d, J=2.3Hz, 1H), 7.09(dd, J=8.0, 2.2Hz, 1H), 6.98(d, J=7.9Hz, 1H) ,6.31(d,J=8.5Hz,1H),3.80–3.71(m,1H),2.92–2.76(m,3H),2.71(t,J=7.6Hz,2H),2.52–2.41(m,2H ),2.35–2.21(m,2H),2.00(s,3H),1.43-1.40(m,2H),...
Embodiment 2
[0132] N-(trans-4-(2-((R)-8-chloro-1-methyl-1,2,4,5-tetrahydro-3H-benzo[d]azepine-3-yl ) ethyl) cyclohexyl) butanamide (I-2) and the preparation of salts thereof
[0133] Using intermediate 6-1 (5.0 mmol) (prepared according to General Method 8) and butyryl chloride (5.5 mmol) as raw materials, according to the preparation method of compound I-1, 1.7 g of white solid I-2 was obtained, with a yield of 89%.
[0134] 1 H NMR (Chloroform-d, δ:ppm) δ7.14(d, J=2.2Hz, 1H), 7.09(dd, J=8.0, 2.2Hz, 1H), 6.98(d, J=8.0Hz, 1H) ,6.30(d,J=8.5Hz,1H),3.80–3.71(m,1H),2.92–2.76(m,3H),2.71(t,J=7.6Hz,2H),2.52–2.41(m,2H ),2.36–2.20(m,4H),1.43-1.40(m,2H), 1.33-1.30(m,5H),1.24–1.21(m,5H),1.14–1.02(m,4H),0.61(t ,J=7.6Hz,3H).
[0135] ESI-MS:391[M+H + ]
[0136] Preparation of Compound I-2 Mesylate
[0137] Using compound I-2 (1.0 mmol) and methanesulfonic acid (1.0 mmol) as starting materials, the synthesis method of compound I-1 hydrochloride was used to obtain 0.42 g of a white solid with a y...
Embodiment 3
[0140] (trans-4-(2-((R)-8-chloro-1-methyl-1,2,4,5-tetrahydro-3H-benzo[d]azepine-3-yl)ethyl Base) cyclohexyl) ethyl carbamate (I-3) and the preparation of salt thereof
[0141] Using intermediate 6-1 (5.0 mmol) (prepared according to General Method 8) and ethyl chloroformate (5.5 mmol) as raw materials, according to the preparation method of compound I-1, 1.8 g of white solid I-3 was obtained, with a yield of 93% .
[0142] 1 H NMR (Chloroform-d, δ: ppm) δ7.13 (d, J = 2.3Hz, 1H), 7.08 (dd, J = 8.0, 2.2Hz, 1H), 7.00 (d, J = 7.9Hz, 1H) ,6.33(d,J=8.5Hz,1H),3.81–3.70(m,1H),2.93–2.78(m,3H),2.73(t,J=7.6Hz,2H),2.54–2.43(m,2H ),2.37–2.22(m,2H),2.01(s,3H),1.44-1.41(m,2H),1.35-1.33(m,3H),1.28–1.25(m,5H),1.17–1.05(m ,4H).
[0143] ESI-MS:393[M+H + ]
[0144] Preparation of compound 1-3 hydrobromide
[0145] Using compound I-3 (1 mmol) and 5% hydrobromic acid (1 mmol) as starting materials, the synthesis method of compound I-1 hydrochloride was used to obtain 0.3 g of white solid w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com