Analysis method of elagolix sodium raw material and synthetic intermediate thereof
A technology for analysis of elagolix sodium and its analysis method, which is applied in the field of analysis of elagolix sodium raw materials and its synthetic intermediates, can solve the problems of lack of reference analysis standards, obstacles to product application and promotion, etc., and achieve accurate confirmation The effect of reaction endpoint, reducing detection cost and ensuring quality controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1. Instruments and conditions:
[0047] High performance liquid chromatography: Agilent 1260Ⅱ;
[0048] Chromatographic column: Kromasil-Eternity-5-C18 250*4.6mm;
[0049] Mobile phase A: 0.01mol / L potassium dihydrogen phosphate solution (containing 0.05% triethylamine, adjusted to pH=6.0 with phosphoric acid);
[0050] Mobile phase B: acetonitrile;
[0051] Mobile phase A: mobile phase B=95:5 (v:v);
[0052] Flow rate: 1.0mL / min;
[0053] Column temperature: 30°C;
[0054] Detection wavelength: 210nm;
[0055] Injection volume: 10uL;
[0056] 2. Implementation steps:
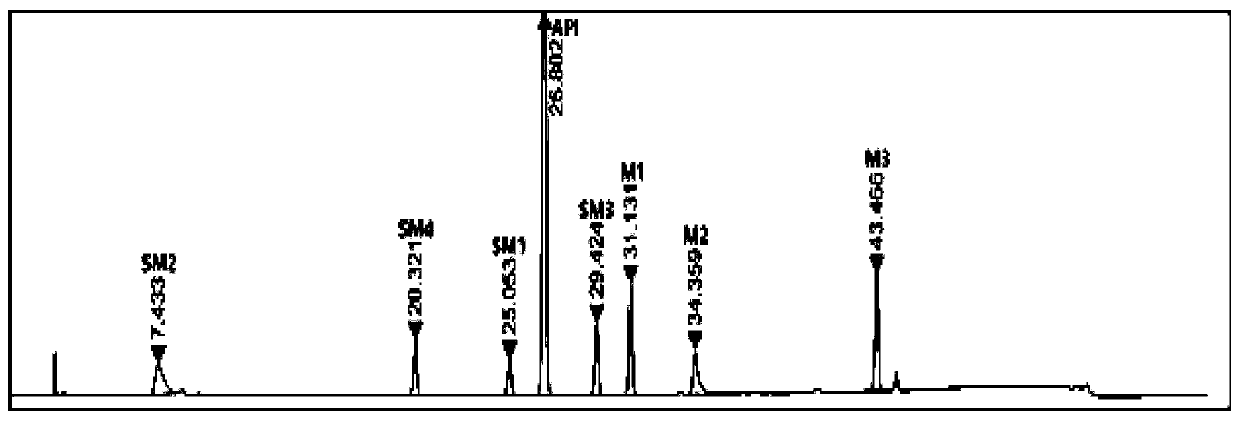
[0057] Weigh 100 mg of elagolix sodium, 10 mg of each intermediate and starting material, respectively, and place in a 100 mL measuring bottle, dissolve and constant volume, as the test solution. Inject 10uL of the above solution into the high performance liquid chromatograph, record the chromatogram, the result is as follows figure 1 .
[0058] Depend on figure 1 It can be seen that the retenti...
Embodiment 2
[0060] 1. Instruments and conditions:
[0061] High performance liquid chromatography: Agilent 1260Ⅱ;
[0062] Chromatographic column: Kromasil-Eternity-5-C18 250*4.6mm;
[0063] Mobile phase A: 0.02mol / L potassium dihydrogen phosphate solution (containing 0.05% triethylamine, adjusted to pH=6.0 with phosphoric acid);
[0064] Mobile phase B: acetonitrile;
[0065] Mobile phase A: mobile phase B=95:5 (v:v);
[0066] Flow rate: 1.0mL / min;
[0067] Column temperature: 30°C;
[0068] Detection wavelength: 210nm;
[0069] Injection volume: 10uL;
[0070] 2. Implementation steps:
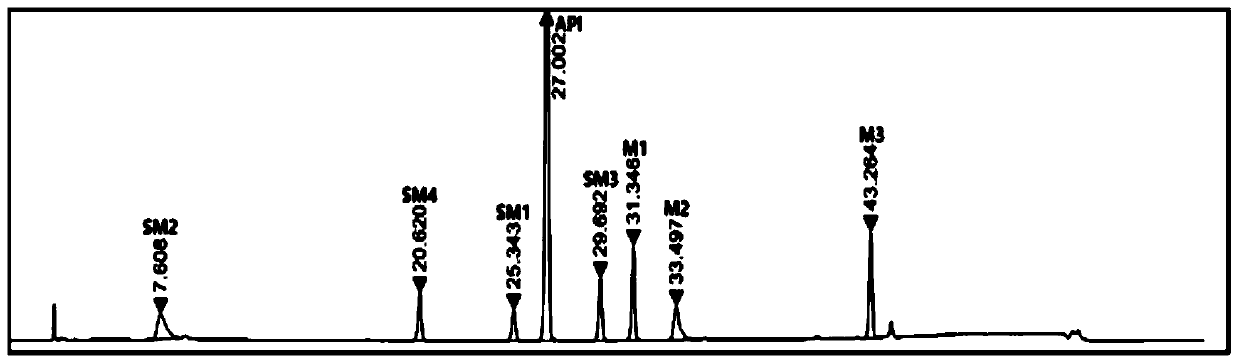
[0071] Weigh 100 mg of elagolix sodium, 10 mg of each intermediate and starting material, respectively, and place in a 100 mL measuring bottle, dissolve and constant volume, as the test solution. Inject 10uL of the above solution into the high performance liquid chromatograph, record the chromatogram, the result is as follows figure 2 .
[0072] Depend on figure 2 It can be seen that the ret...
Embodiment 3
[0074] 1. Instruments and conditions:
[0075] High performance liquid chromatography: Agilent 1260Ⅱ;
[0076] Chromatographic column: Kromasil-Eternity-5-C18 250*4.6mm;
[0077] Mobile phase A: 0.01mol / L potassium dihydrogen phosphate solution (containing 0.05% triethylamine, adjusted to pH=6.0 with phosphoric acid);
[0078] Mobile phase B: acetonitrile;
[0079] Mobile phase A: mobile phase B=95:5 (v:v);
[0080] Flow rate: 1.0mL / min;
[0081] Column temperature: 40°C;
[0082] Detection wavelength: 210nm;
[0083] Injection volume: 10uL;
[0084] 2. Implementation steps:
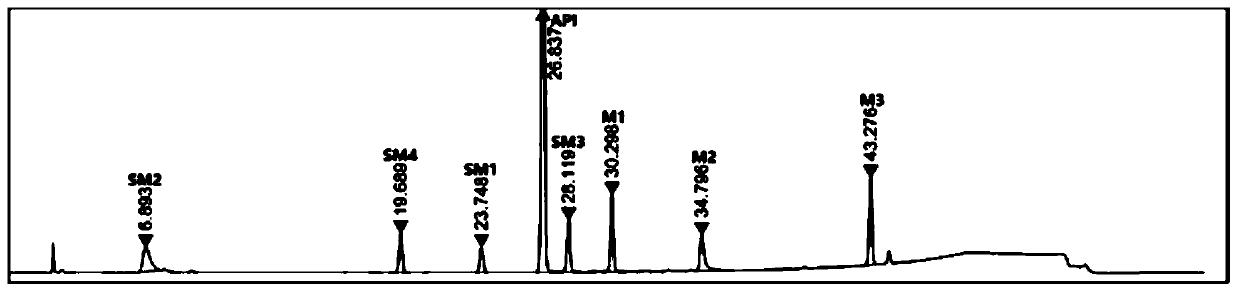
[0085] Weigh 100 mg of elagolix sodium, 10 mg of each intermediate and starting material, respectively, and place in a 100 mL measuring bottle, dissolve and constant volume, as the test solution. Inject 10uL of the above solution into the high performance liquid chromatograph, record the chromatogram, the result is as follows image 3 .
[0086] Depend on image 3 It can be seen that the reten...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com