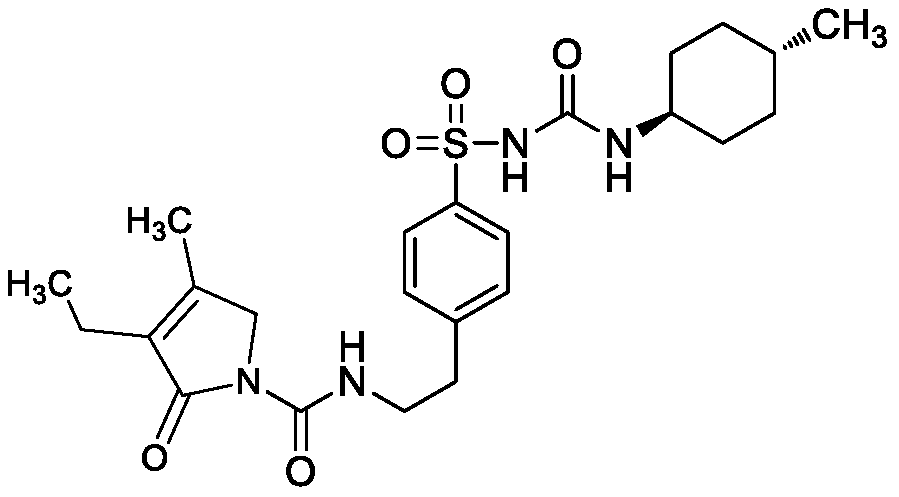
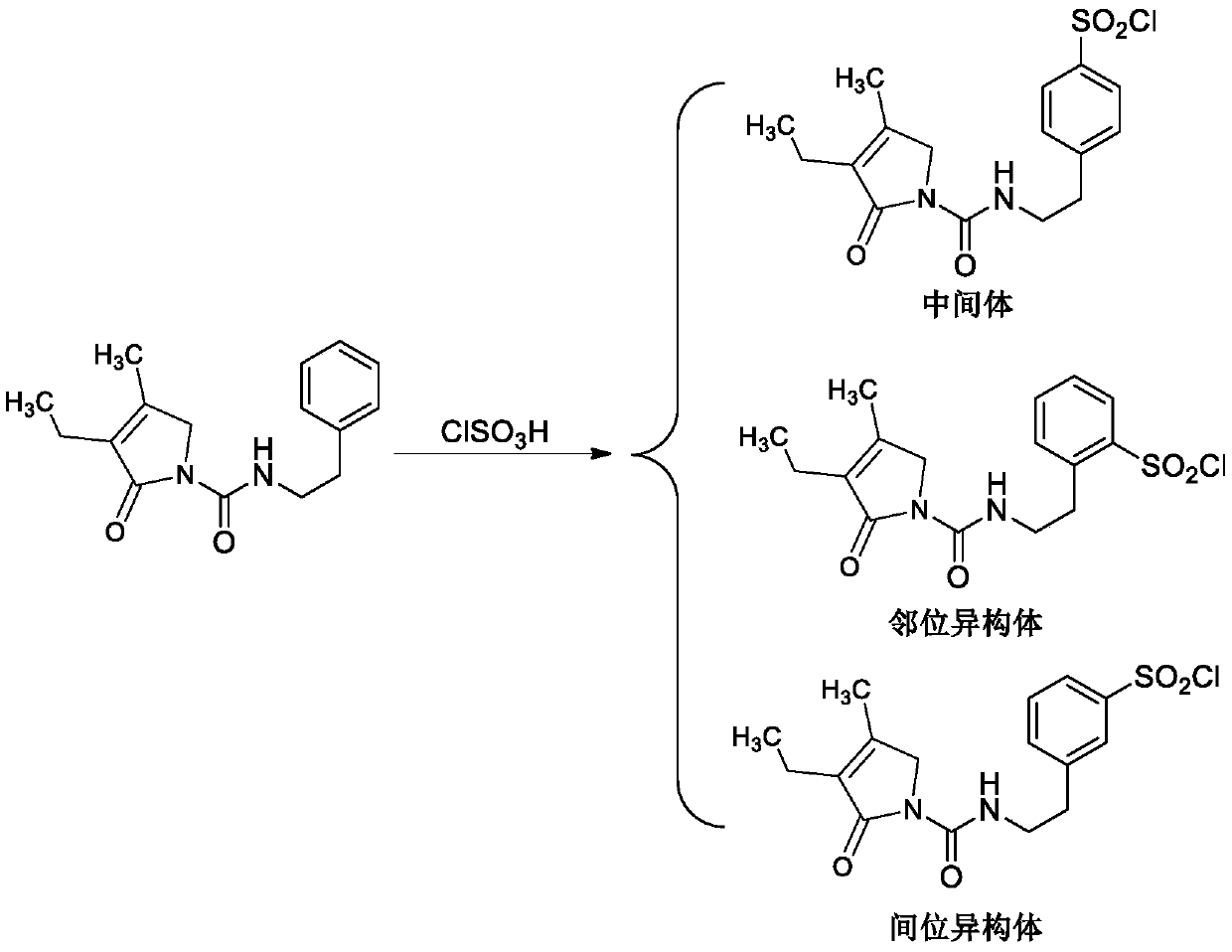
Separation method and synthesis method of glimepiride intermediate and isomers thereof
A technology of separation method and synthesis method, which is applied in the field of separation method and synthesis of glimepiride intermediates and its isomers, can solve the problems of not finding a separation method for benzenesulfonyl chloride, and achieve the effect of quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Glimepiride intermediate and its isomers (4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl]benzene Separation method of ortho-isomer and meta-isomer of sulfonyl chloride
[0040] 4-[2-(3-ethyl-4-methyl-2-oxygen-3-pyrroline-1-carboxamido) ethyl] benzenesulfonyl chloride (intermediate ) and its ortho-isomers and meta-isomers are separated, and the chromatographic conditions are as follows:
[0041] Chromatographic column: Agilent Poroshell 120 EC-C18 4.6×150mm, 4μm
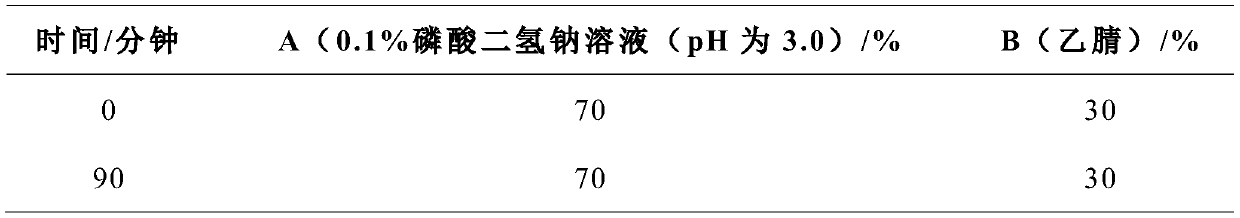
[0042] Mobile phase: A (0.1% sodium dihydrogen phosphate solution (adjust the pH value to 3.0±0.5 with phosphoric acid))-B (acetonitrile)=70:30;
[0043] Detection wavelength: 228nm;
[0044] Column temperature: 30°C;
[0045] Flow rate: 1.0mL / min;
[0046] Isocratic elution:
[0047]
[0048] The separation method provided in this application can well separate the ortho isomer and the meta isomer.
Embodiment 2
[0049] Example 2 Glimepiride intermediate and its isomer (4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl]benzene Synthetic method of sulfonyl chloride (intermediate) and its ortho isomer and meta isomer)
[0050] Add 27.2g (0.10mol) 3-ethyl-4-methyl-2-oxo-3-pyrroline-1-N-(2-phenylethyl)-formamide to the flask, 34.95g (0.30mol) chlorosulfonic acid ), the addition was completed, and the reaction was stirred at 80°C for 5h, and the HPLC monitoring reaction was complete;
[0051] Add the reaction liquid to 200g of ice water, stir to precipitate a white solid, filter, wash the filter cake with water to obtain white crystals, and dry at 50°C for 12h; to obtain 4-[2-(3-ethyl-4-methyl-2-oxo- 3-pyrroline-1-carboxamido)ethyl]benzenesulfonyl chloride and its ortho and meta isomers were 26.2g in total;
[0052] The white crystals were separated by preparative high-performance liquid chromatography, and the chromatographic conditions were as follows:
[0053] Chromatograph...
Embodiment 3
[0062] Example 3 Glimepiride intermediate and its isomer (4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl]benzene Synthetic method of sulfonyl chloride (intermediate) and its ortho isomer and meta isomer)
[0063] Add 27.2g (0.10mol) 3-ethyl-4-methyl-2-oxo-3-pyrroline-1-N-(2-phenylethyl)-formamide to the flask, 69.9g (0.60mol) of chlorosulfonic acid ), the addition was completed, and the reaction was stirred at 100°C for 2h, and the HPLC monitoring reaction was complete;
[0064] Add the reaction liquid to 210g of ice water, stir to precipitate a white solid, filter, wash the filter cake with water to obtain white crystals, and dry at 50°C for 15h to obtain 4-[2-(3-ethyl-4-methyl-2-oxo- 3-pyrroline-1-carboxamido)ethyl]benzenesulfonyl chloride and its ortho and meta isomers were 28.8g in total;
[0065] The white crystals were separated by preparative high-performance liquid chromatography, and the chromatographic conditions were as follows:
[0066] Chromatogra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com