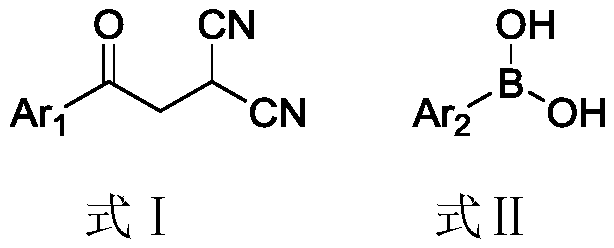
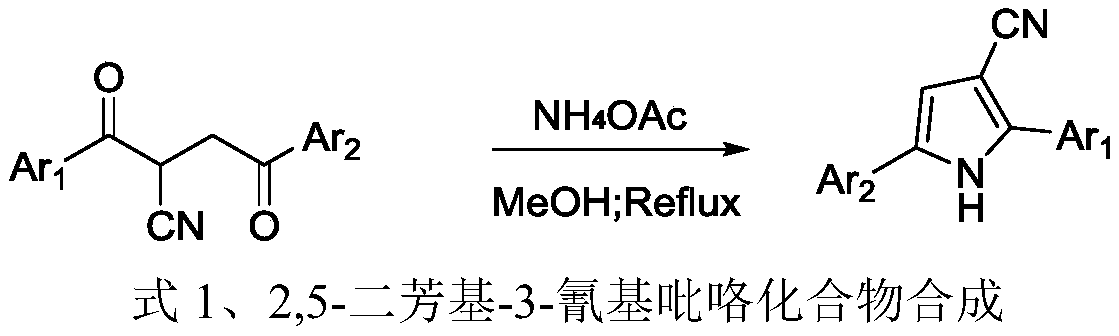
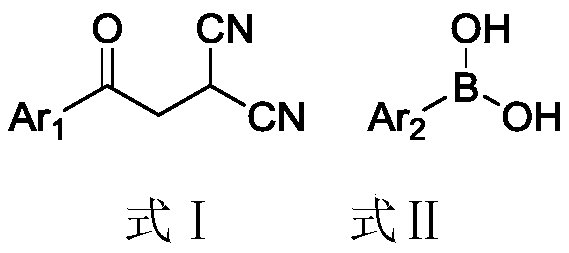
Production method of 2,5-diaryl-3-cyanopyrrole compound
A cyanopyrrole and compound technology, which is applied in the field of preparation of 2,5-diaryl-3-cyanopyrrole compounds, can solve the problems such as the difficulty in obtaining ketone compounds, and achieves overcoming the difficulty in obtaining raw materials, novel synthesis methods, and the like. high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1, 2-p-tolyl-3-cyano-5-phenylpyrrole (m1)
[0023] Add 184mg (1.0mmol) of 2-phenylacetylmalononitrile, 204mg (1.5mmol) of p-tolueneboronic acid, 44.8mg (0.2mmol) of palladium acetate, and 15.0ml of toluene into a 50ml three-necked flask, and heat up to 100°C under stirring to react for 24 After 1 hour, the reaction was detected by TLC (petroleum ether:ethyl acetate=5:1 volume ratio, when the complete disappearance of the raw material 2-phenylacetylmalononitrile was detected, the reaction was judged to be complete).
[0024] After the reaction, the resulting reaction solution was suction filtered through diatomaceous earth, concentrated by a rotary evaporator (evaporating temperature of 40°C) to remove toluene, and concentrated liquid column chromatography (petroleum ether: ethyl acetate = 20:1 volume ratio) 222.2 mg of the product 2-p-tolyl-3-cyano-5-phenylpyrrole was obtained with a yield of 86%.
[0025] The column chromatography is specifically as follows: p...
Embodiment 2
[0044] Example 2, 2,5-di-p-tolyl-3-cyanopyrrole (m2)
[0045] Replace 2-phenylacetylmalononitrile with 2-p-methylphenylacetylmalononitrile, the molar weight remains unchanged, and the rest are equal to Example 1. 227.7 mg of the white solid product 2,5-xylyl-3-cyanopyrrole was obtained, with a yield of 84% and a purity of 97%.
[0046] Its structural formula is:
[0047]
[0048] White solid; mp:197.3-198.0℃; 1 H NMR (500MHz, DMSO-d 6 )δ12.06(s,1H),7.74(d,J=8.5Hz,2H),7.70(d,J=8.0Hz,2H),7.35(d,J=8.0Hz,2H),7.24(d, J=8.0Hz, 2H), 6.98(d, J=2.5Hz, 1H), 2.37(s, 3H), 2.32(s, 3H); HRMS(ESI): m / z calcd for C 19 h 17 N 2 [M+H] + :273.1386,found:273.1389.
Embodiment 3
[0049] Example 3, 2,5-diphenyl-3-cyanopyrrole (m3)
[0050] Replace p-tolueneboronic acid with phenylboronic acid, the molar weight is constant, and all the other are the same as in Example 1. 205.4 mg of the white solid product 2,5-diphenyl-3-cyanopyrrole was obtained, with a yield of 84% and a purity of 97%.
[0051] Its structural formula is:
[0052]
[0053] White solid; mp:206.1-206.8℃; 1 H NMR (500MHz, DMSO-d 6 )δ12.21(s,1H),7.85(d,J=7.5Hz,2H),7.82(d,J=7.0Hz,2H),7.56(t,J=8.0Hz,2H),7.48–7.40( m,3H),7.31(t,J=7.0Hz,1H),7.09(d,J=2.5Hz,1H); HRMS(ESI):m / z calcd for C 17 h 13 N 2 [M+H] + :245.1073,found:245.1074.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com