Method for preparing isoxazolo-isoquinolinone derivative under electro-catalysis
A technology of isoquinolone and isoxazole, which is applied in the field of preparation of isoxazoloisoquinolinone derivatives under electrocatalysis, can solve problems such as toxic solvents, long reaction time, and use of metal catalysts, so as to avoid Side reactions, simple synthesis method, fast response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of Isoxazoloisoquinolinone Derivative 2a
[0023]
[0024] Add N-alkoxybenzamide 1a (0.1mmol, 26.5mg) and tetra-n-butyl ammonium hexafluorophosphate (0.1mmol, 38.7mg) into a 10mL electrolytic cell without a diaphragm, and both cathode and anode electrodes use graphite felt electrodes ( 2cm x 1cm x 0.5cm). Afterwards, the system was filled with nitrogen, and 95% ethanol (5 mL) was added. Electrolysis was carried out at 80°C and a constant current of 2mA, and the reaction was carried out for 4h. After the reaction is complete, use a rotary evaporator to remove the solvent to obtain a crude product, which is separated by column chromatography (200-300 mesh silica gel, petroleum ether / ethyl acetate=1 / 1), and use a rotary evaporator to remove the solvent to obtain the target product The yield of the unsubstituted isoxazoloisoquinolinone derivative 2a is 93%.
[0025] Spectral analysis data 2a:
[0026] 1 H NMR (CDCl 3 ,400MHz):δ8.54(d,J=7.1Hz,1H),7.59-7....
Embodiment 2
[0028] Replace 1a in Example 1 with 1b, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0029]
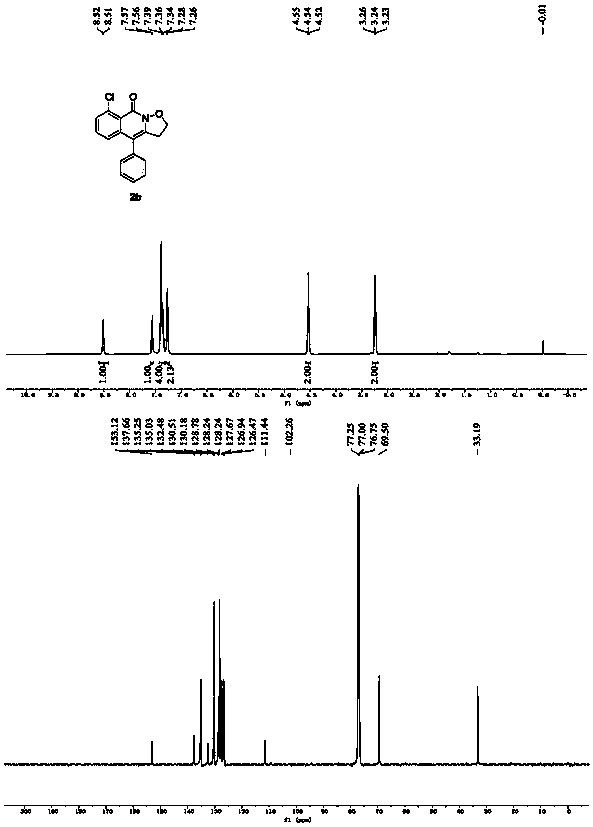
[0030] Spectrum analysis data 2b:
[0031] 1 H NMR (500MHz, CDCl 3 ):δ8.51(d,J=8.0Hz,1H),7.56(d,J=7.6Hz,1H),7.40-7.32(m,4H),7.27(d,J=6.7Hz,2H),4.54 (t, J=7.6Hz, 2H), 3.24(t, J=7.6Hz, 2H); 13 C NMR (125MHz, CDCl 3 )δ153.12, 137.66, 135.25, 135.03, 132.48, 130.51, 130.18, 128.78, 128.24, 127.67, 126.94, 126.47, 111.44, 69.50, 33.19.
Embodiment 3
[0033] Replace 1a in Example 1 with 1c, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0034]
[0035] Spectrum analysis data 2c:
[0036] 1 H NMR (500MHz CDCl 3 ):δ8.47(d,J=3.6Hz,1H),7.53-7.49(m,2H),7.48–7.42(m,2H),7.34-7.28(m,3H),4.58(t,J=7.6 Hz,2H),3.39(t,J=7.6Hz,2H); 13 C NMR (125MHz, CDCl 3 ): δ152.76, 134.51, 134.43, 132.89, 132.53, 132.02, 130.14, 129.01, 128.29, 127.29, 126.71, 126.34, 112.60, 69.69, 32.36
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com