Preparation and application of hydrogen peroxide near-infrared fluorescent probe
A fluorescent probe and hydrogen peroxide technology, applied in the field of fluorescent probes, can solve the problems of short emission wavelength and small Stokes shift of fluorescent probes, and achieve the effect of good spectral response performance and rapid response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of fluorescent probes
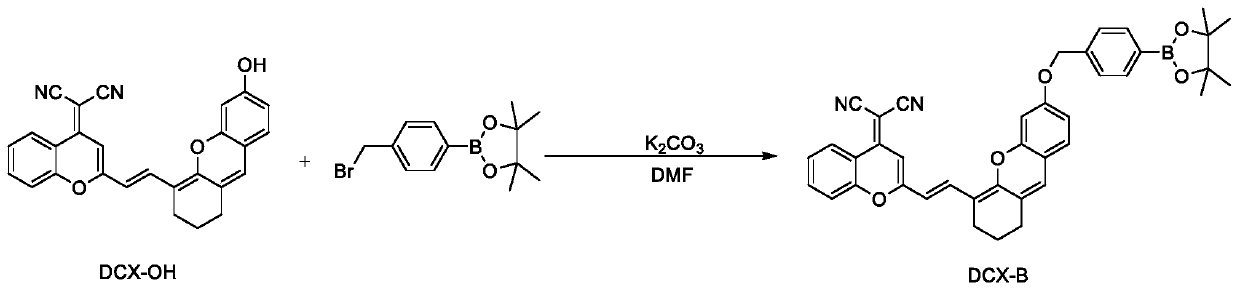
[0027] Synthetic route such as figure 1 . in N 2 Under protection, in a 50mL round bottom flask, DCX-OH (83.6mg, 0.2mmol), 4-bromomethylphenylboronic acid pinacol ester (71.2mg, 0.24mmol), potassium carbonate (55.2mg, 0.4mmol) Dissolved in 5.0 mL of DMF and the reaction was stirred at 60 °C for 4 h. After the reaction is complete, dilute with water and wash with CH 2 Cl 2 For extraction, the organic layer was collected, washed with brine, anhydrous Na 2 SO 4 Dry, filter and concentrate. Crude product with CH 2 Cl 2 The eluent was subjected to column chromatography to obtain a dark blue solid product DCX-B (54.5 mg, yield 43%), which was a fluorescent probe. 1 H NMR (400MHz, CDCl 3 )δ=8.89(d,J=8.3,1H),8.09(d,J=15.4,1H),7.88(d,J=7.8,2H),7.74(t,J=7.8,1H),7.53(d ,J=8.3,1H),7.47(d,J=7.8,2H),7.39(d,J=7.8,1H),7.02(d,J=8.3,1H),6.75-6.66(m,3H), 6.48(s,1H),6.06(d,J=15.4,1H),5.16(s,2H),2.61-2.50(m,2H),2.46(t,J=5.6,2H),1.87-1.81(m ,2H...
Embodiment 2
[0029] Fluorescent probes and H 2 o 2 Solution preparation
[0030] Preparation of probe solution: Weigh a certain amount of probe solid and dissolve it in DMSO to make 1×10 -4 M probe solution. h 2 o 2 Solution preparation: measure a certain amount of H with a volume fraction of 30% 2 o 2 solution, and then dilute it to a 50mL volumetric flask with distilled water to make 1×10 -2 M detection solution. will be 1.0×10 -2 M of H 2 o 2 The solution was gradually diluted to obtain 1.0 × 10 -5 -2.0×10 -3 M of H 2 o 2 solution. Mix 1.0 mL of probe stock solution and 1.0 mL of HO 2 o 2 The solution was added to a 10mL volumetric flask, and after constant volume with buffer solution, the concentration was 1.0×10 -5 M fluorescent probes and 1.0 x 10 -6 -2.0×10 -4 M of H 2 o 2 Mix the solutions to be tested.
Embodiment 3
[0032] Fluorescent probes with H 2 o 2 Determination of the fluorescence spectrum of the action
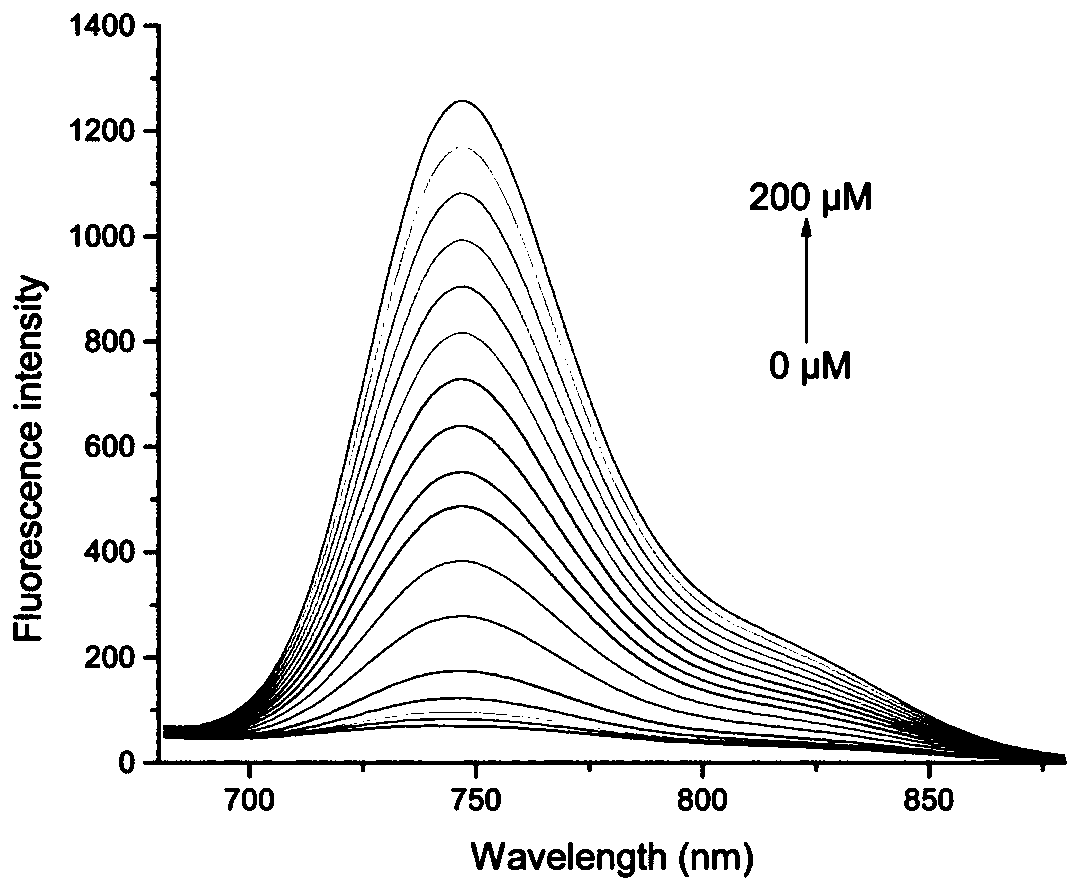
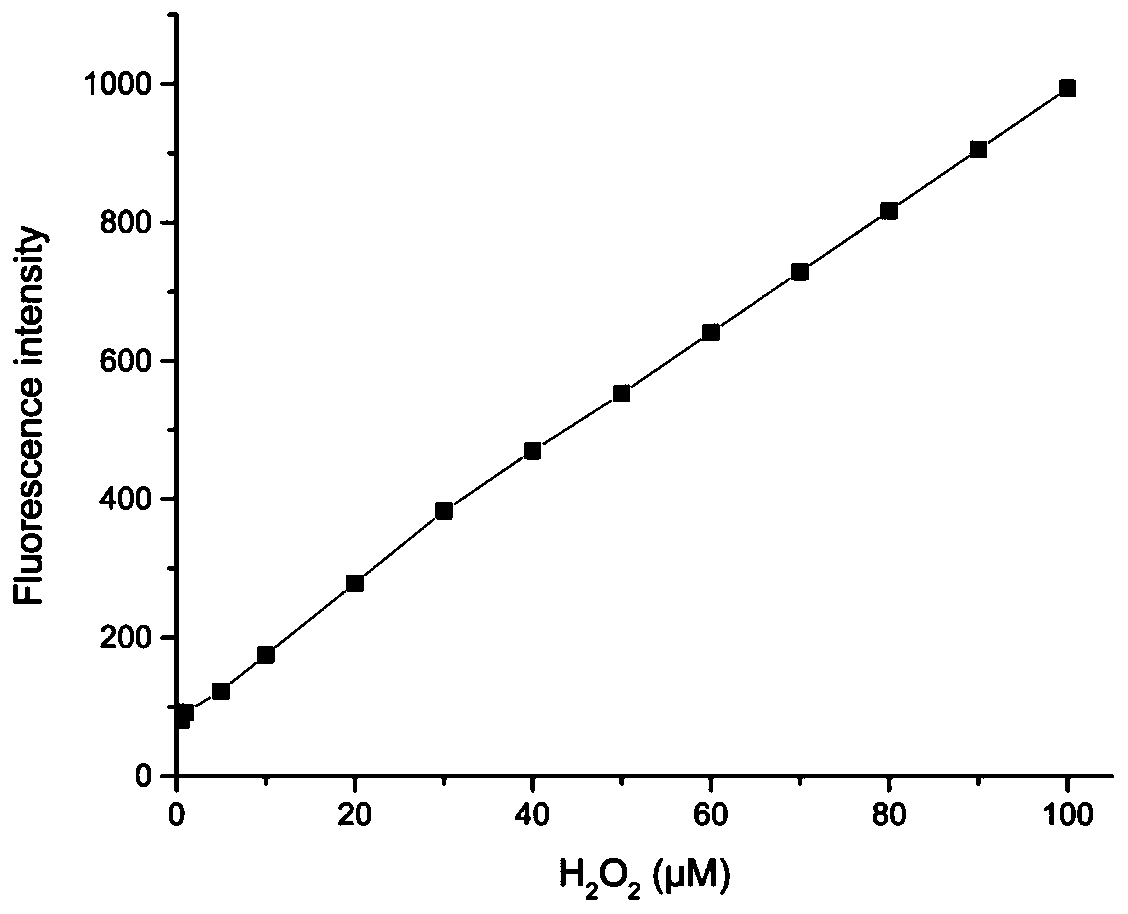
[0033] figure 2 as a fluorescent probe with H 2 o 2 The fluorescence spectrum of the action, the concentration of the fluorescent probe is 10 μM, H 2 o 2 The concentrations are 0, 0.5, 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 120, 150, 200 μM. The excitation wavelength used in the experiment is 590nm, and the emission wavelength range is 680-900nm. The slit width is 10.0 nm / 10.0 nm, and the fluorescence measurement instrument used is a Hitachi F4600 fluorescence spectrophotometer. from figure 2 It can be seen that adding H 2 o 2 Before, due to the quenching effect of boronate, the fluorescent probe had almost no fluorescence emission peak; adding H 2 o 2 Afterwards, a near-infrared fluorescence emission peak appeared in the near-infrared region (748nm). This is because the probe molecules are H 2 o 2 Oxidation, resulting in the breakage of borate ester bonds...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com