Bispecific antibody and preparation method and application thereof
A bispecific antibody and heterodimer technology, applied in the direction of antibodies, chemical instruments and methods, antibody mimics/scaffolds, etc., can solve the problem of short half-life, small molecular weight, reduced production complexity and easy penetration through tissues and Tumor cells reach the target and other issues, to achieve the effect of high safety and efficient tumor killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Embodiment 1: expression vector construction
[0110] (1) Sequence design of CD20×CD3 BsAb
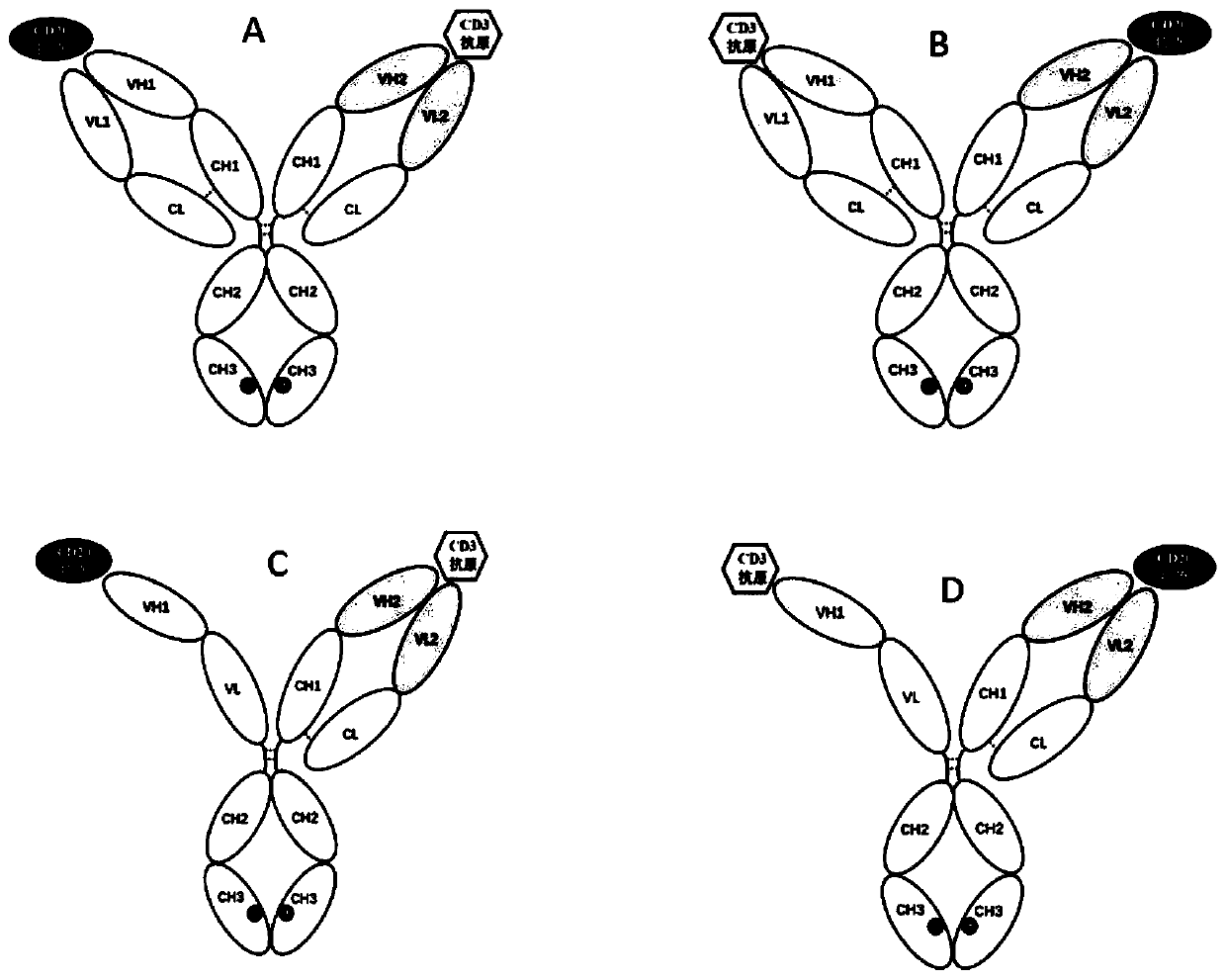
[0111] The bispecific antibody in the practice of the present invention comprises 3 or 4 polypeptide chains ( figure 1 ), respectively named as the first heavy chain (its variable region has the amino acid sequence shown in SEQ ID No.: 2), the first light chain (its variable region has the amino acid sequence shown in SEQ ID No.: 4), the first Second heavy chain (its variable region has the amino acid sequence shown in SEQ ID No.: 6), the second light chain (its variable region has the amino acid sequence shown in SEQ ID No.: 10) and scFv-Fc chain (having the amino acid sequence shown in SEQ ID No.: 10) The amino acid sequence shown in SEQ ID No.:11, SEQ ID No.:12, SEQ ID No.:13 or SEQ ID No.:14; or its variable region has SEQ ID No.:2, SEQ ID No.:4 , SEQ ID No.:6, SEQ ID No.:8 or the amino acid sequence shown in SEQ ID No.:10), forming an immunoglobulin domain specifically bi...
Embodiment 2
[0119] Example 2: Transient transfection and expression of bispecific antibodies
[0120]The endotoxin-free plasmid maxi kit (Endo-Free-Plasmid Maxi Kit (100), purchased from OMEGA, catalog number D6926-04) was used for massive extraction of plasmids, and the operation steps were performed according to the instructions provided by the kit. HEK293 cells were cultured to a cell density of 2.0-3.0×10 6 cells / mL, the cell suspension was centrifuged for 5min at a speed of 1000rpm, the old culture supernatant was discarded, and the cells were resuspended with fresh medium (OPM-291CD05Medium, purchased from Shanghai OPM Biotechnology Co., Ltd.) to a density of 1.0×106 / mL. Co-transfection was carried out according to the plasmid combinations provided in Table 1, and the transfected cell suspension was placed in a culture shaker at 37°C, 5% CO2, and 120rpm for 5-7 days in the dark, and supplemented with supplements on the 4th day. material.
[0121] Table 1 Transient transfection of...
Embodiment 3
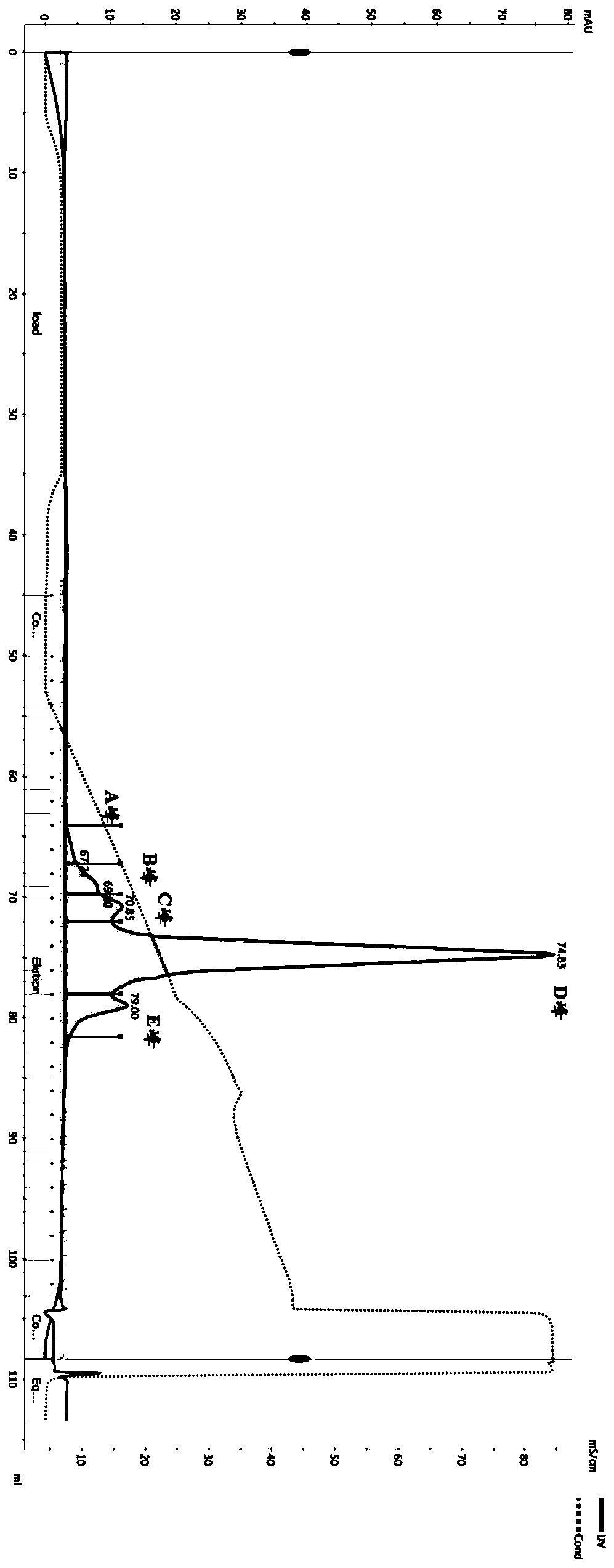
[0124] Example 3 Purification of double antibodies
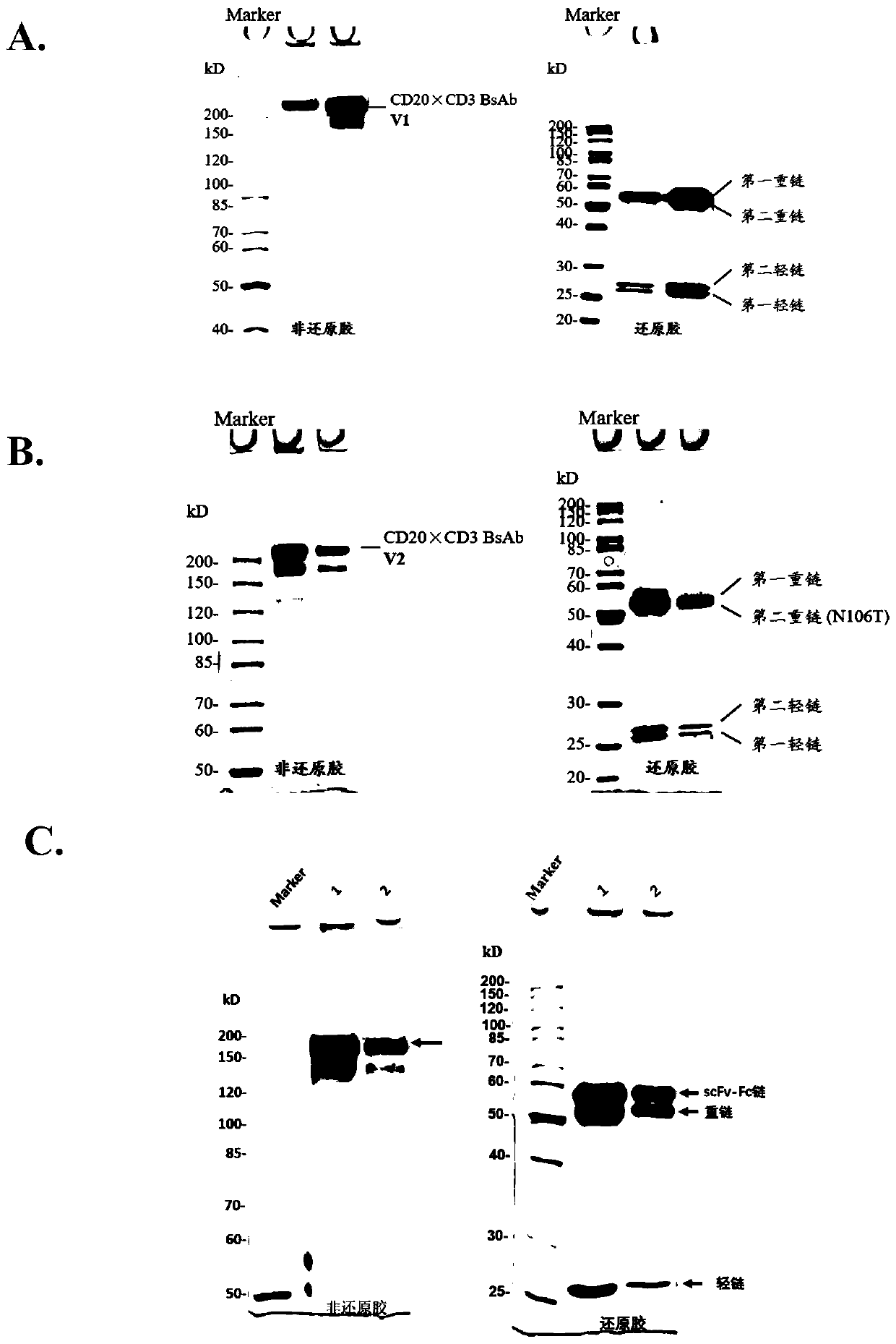
[0125] The expression supernatant was collected by centrifugation, the cell supernatant was filtered with a 0.22 μm filter membrane, and the protein A affinity chromatography filler (MabSelect SuRe TM , purchased from GE Healthcare, Cat. No. 17-5438-02) to capture the BsAb protein in the supernatant, using equilibration buffer (137mM NaCl, 2.7mM KCl, 10mM NaCl 2 HPO 4 , 1.8mM KH 2 PO 4 ) after washing away non-specifically bound proteins (about 10 column volumes), elute with elution buffer (100mM glycine, pH 3.0-pH 3.5) for 5-10 column volumes, collect the eluate, and wash with neutralization buffer solution (1M Tris-HCl, pH 9.0) to adjust the pH to neutral. Eluted proteins were analyzed by SDS-PAGE. figure 2 -A, 2-B show that after one-step purification of the four-chain bispecific antibody by protein A, both V1 and V2 can reach high purity, and reducing electrophoresis shows that the ratio of the two heavy chains to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com