Preparation method of aromatic thioether compound
A technology of aromatic sulfides and compounds, applied in the field of catalysis, can solve the problems of pollution and high cost, and achieve the effect of high reaction efficiency, low cost and good application prospect of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
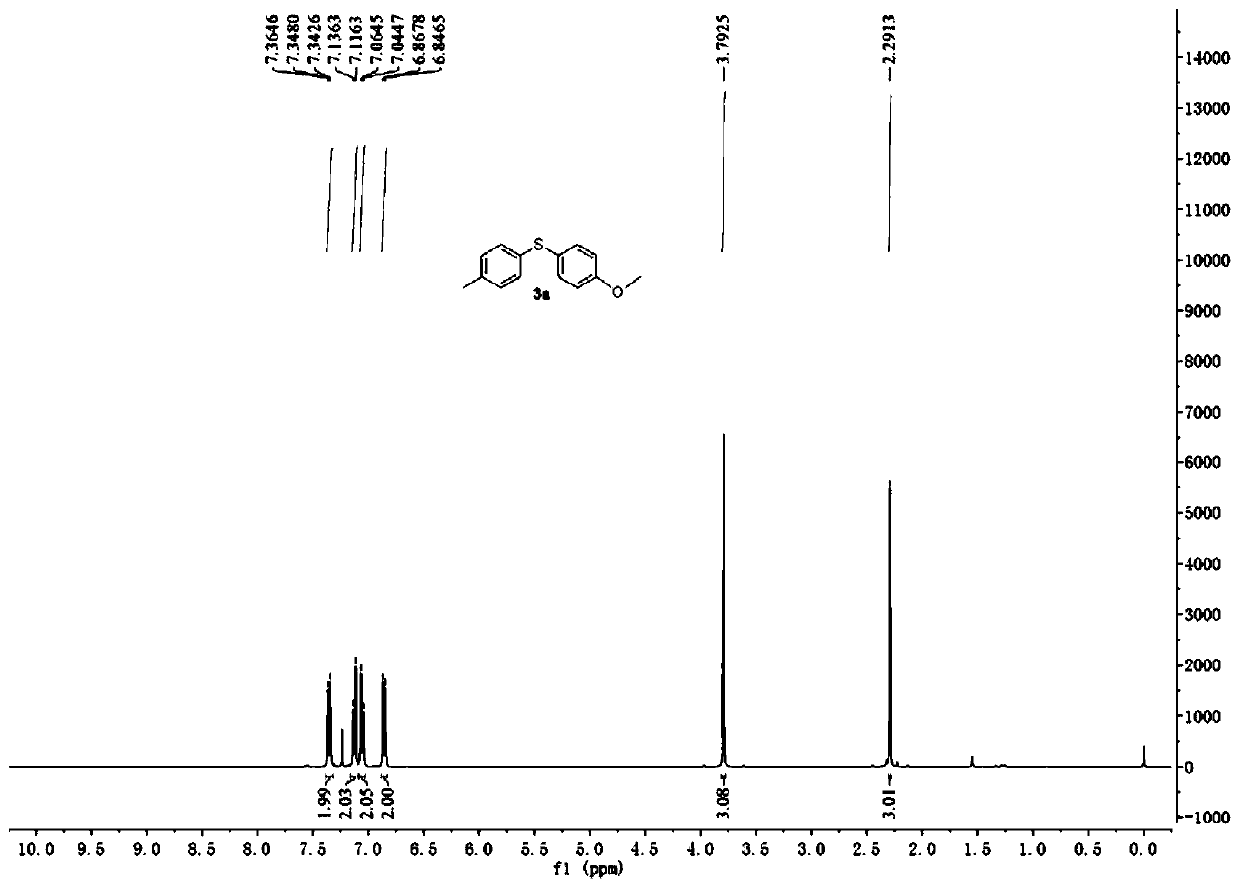
[0045] Embodiment 1. Catalyzed synthesis of 4-methoxy-4'-methyl-diphenylsulfide by cercosporin catalyst
[0046] Add cercosporin (0.005mmol), p-methoxybenzenetetrafluoroborate diazonium salt (0.6mmol), p-methylthiophenol (0.5mmol), 2mL DMSO in a 10mL reaction tube in turn, and then , 15W white light irradiation, room temperature 25 ℃ reaction 20h. The reaction solution was washed three times with water, and the organic phase was collected and then dried over anhydrous magnesium sulfate. After filtration, the solvent was evaporated to dryness by rotary evaporation, and the thin-layer silica gel plate of 300-500 mesh was used for rapid separation, and the eluent used was ethyl acetate / petroleum ether (v:v=3:100) to obtain 4-methoxy-4' -Methyl-diphenyl sulfide, the productive rate is 87%, the product 1 H-NMR spectrum see figure 1 .
Embodiment 2
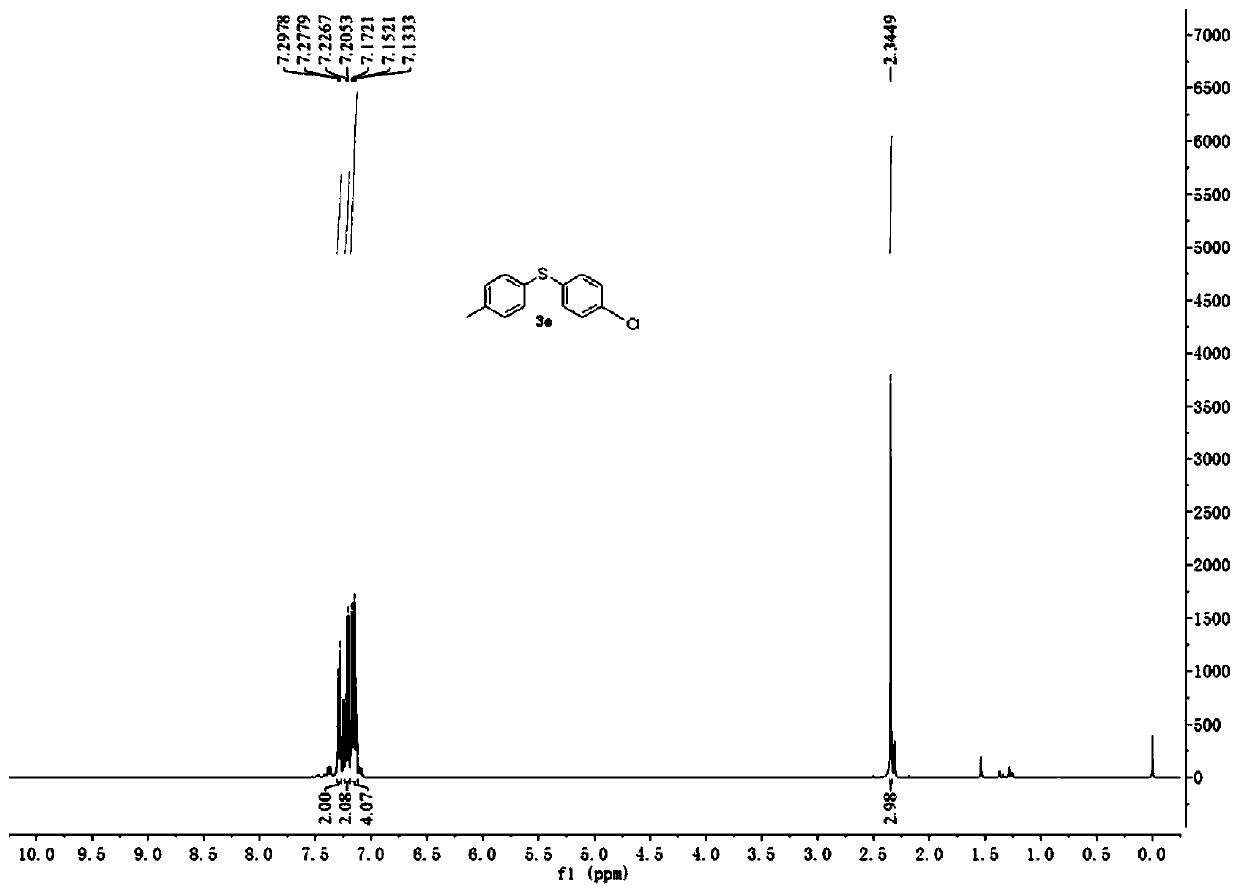
[0047] Embodiment 2. Catalyzed synthesis of 4-chloro-4'-methyl-diphenyl sulfide by cercosporin catalyst
[0048] Add cercosporin (0.005mmol), p-chlorobenzenetetrafluoroborate diazonium salt (0.6mmol), p-methylthiophenol (0.5mmol), 2mL DMSO in a 10mL reaction tube in turn, and then in air, 15W White light irradiation, room temperature 25 ℃ reaction 20h. The reaction solution was washed three times with water, and the organic phase was collected and then dried over anhydrous magnesium sulfate. Filtrate, dry the solvent by rotary evaporation, and then use a 300-500 mesh thin-layer silica gel plate for rapid separation. The eluent used is ethyl acetate / petroleum ether (v:v=3:100) to obtain 4-chloro-4'-formazan Base-diphenyl sulfide, the productive rate is 65%, the product 1 H-NMR spectrum see figure 2 .
Embodiment 3
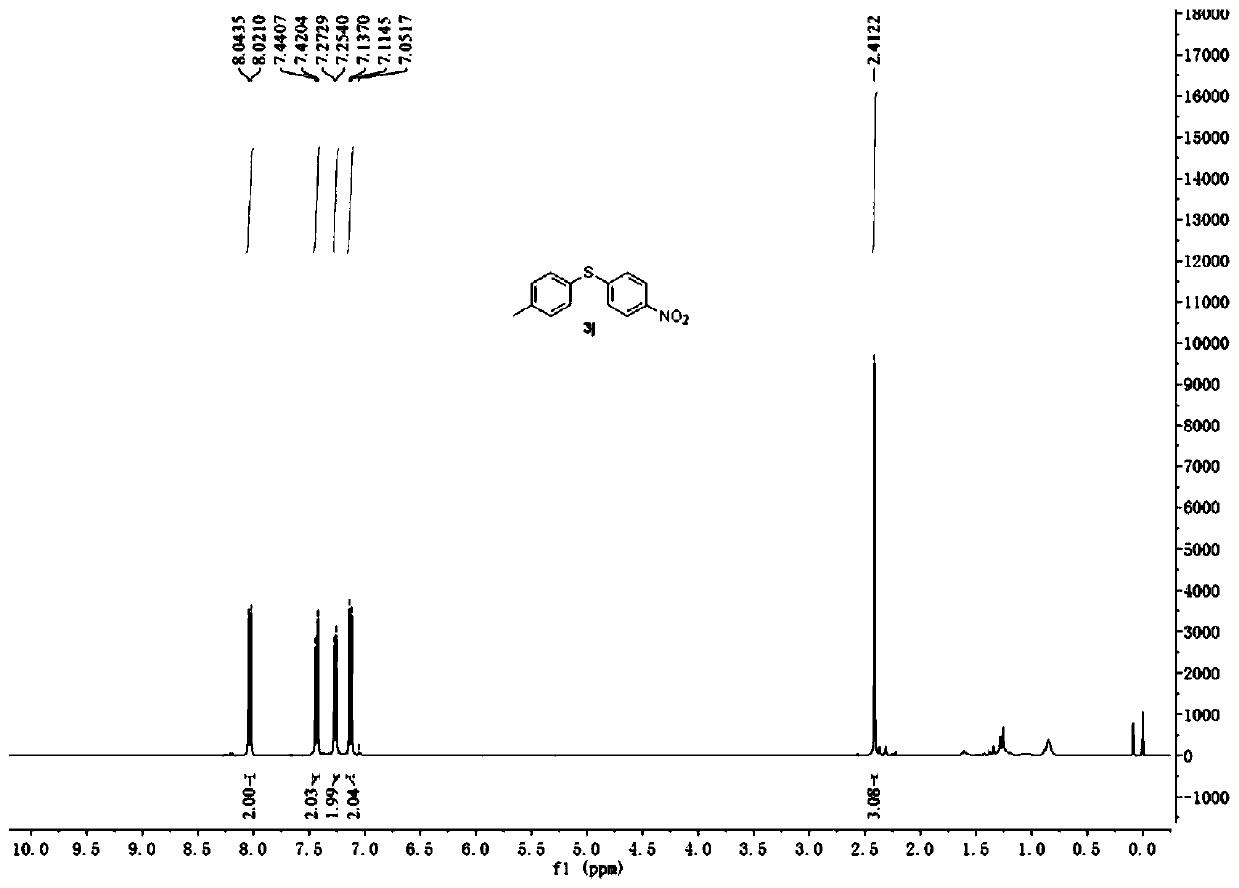
[0049] Embodiment 3. Catalyzed synthesis of 4-nitro-4'-methyl-diphenyl sulfide by cercosporin catalyst
[0050]Add cercosporin (0.005mmol), p-nitrobenzenetetrafluoroborate diazonium salt (0.6mmol), p-methylthiophenol (0.5mmol), 2mL DMSO successively into a 10mL reaction tube, and then in air, 15W white light irradiation, room temperature 25 ℃ reaction 20h. The reaction solution was washed three times with water, and the organic phase was collected and then dried over anhydrous magnesium sulfate. After filtration, the solvent was evaporated to dryness by rotary evaporation, and then separated quickly with a 300-500 mesh thin-layer silica gel plate, and the eluent used was ethyl acetate / petroleum ether (v:v=3:100) to obtain 4-nitro-4'- Methyl-diphenyl sulfide, productive rate is 80%, the product 1 H-NMR spectrum see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com