A kind of amide compound and its preparation method and application
A technology of amide compounds and compounds, applied in the field of amide compounds and their preparation, can solve problems such as lack of practicability and economic value, difficulty in conversion or elimination, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
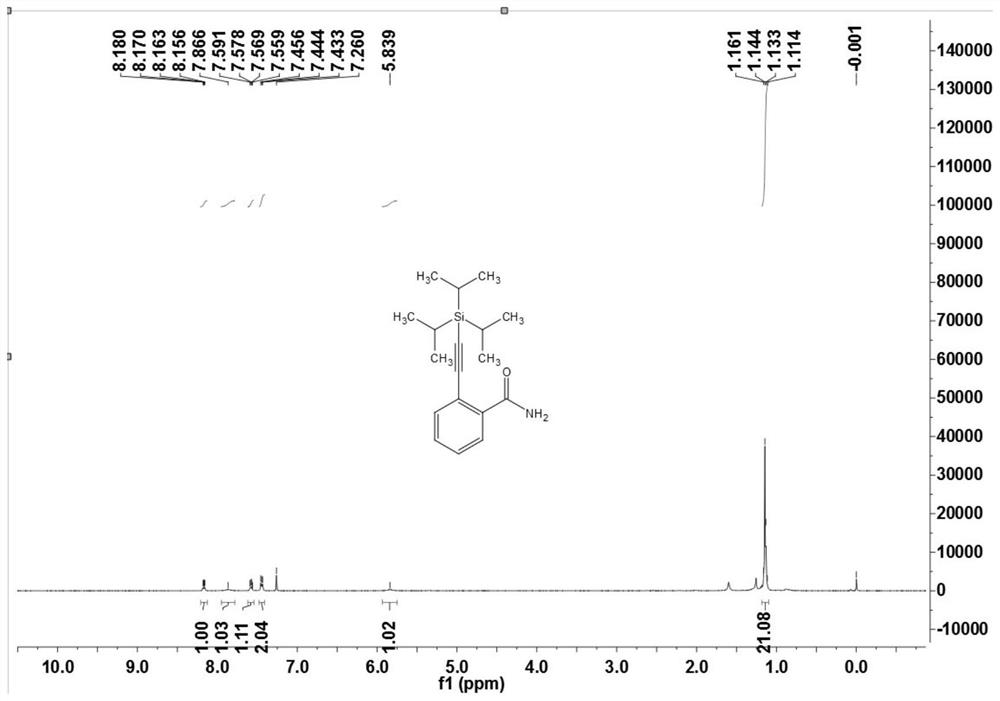
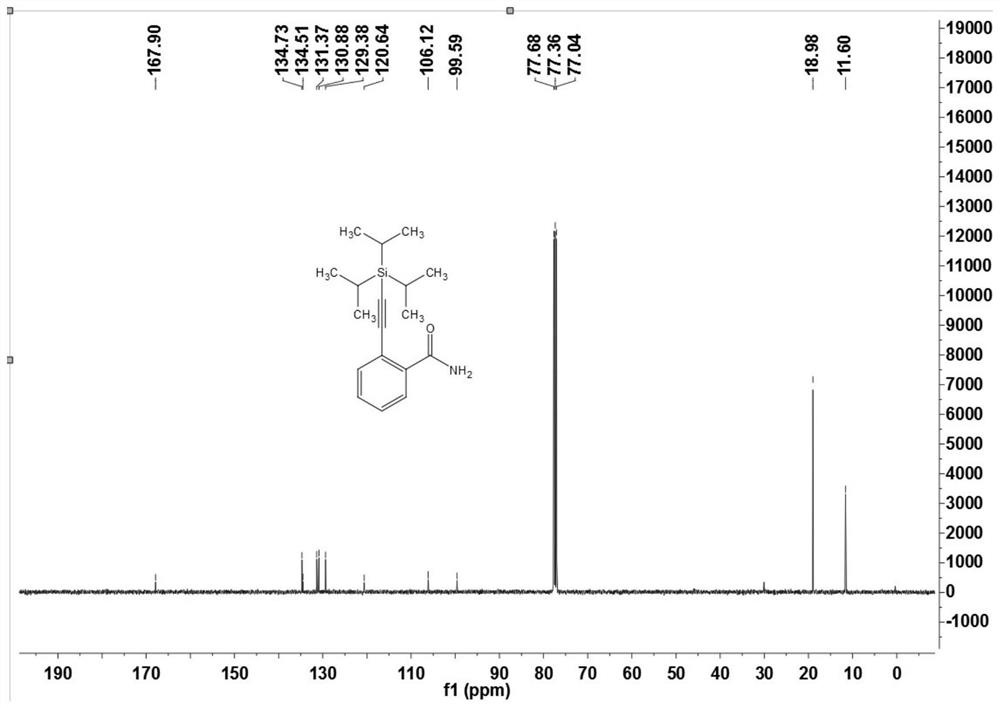
[0107] The preparation of embodiment one 2-((triisopropylsilyl) acetylene) benzamide (3a)
[0108]
[0109] Under nitrogen atmosphere, arylsulfonamide compound 1a (12.1 mg, 0.10 mmol), alkynyl bromide or alkyne 2 (20 μL, 0.10 mmol), dichloro(pentamethylcyclopentadiene base) iridium dimer (2.3mg, 0.0025mmol) or dichloro(p-methylcumene) ruthenium dimer (1.5mg, 0.0025mmol), bis(trifluoromethanesulfonyl)imide silver ( 4.2mg, 0.015mmol) or silver hexafluoroantimonate (5.2mg, 0.015mmol), cesium acetate (30mg, 0.36mmol), (when the reaction uses an alkyne bromide, no additional oxidant is needed; when the reaction uses a terminal alkyne directly, You need 2 equivalents of silver acetate), 1,2-dichloroethane (DCE, 1mL), and react at 120°C for 12 hours. After the reaction was completed, it was cooled to room temperature, filtered through diatomaceous earth, and concentrated to obtain a crude product. The crude product was separated by chromatography on a prepared silica gel plate, ...
Embodiment 2
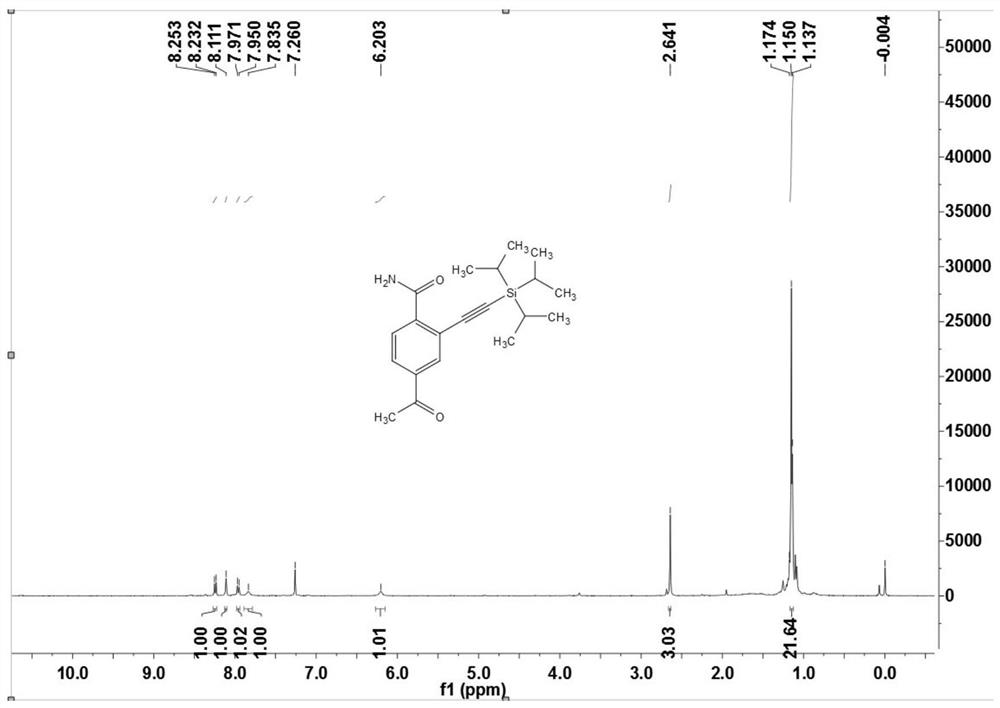
[0112] Preparation of Example Two 4-acetyl-2-((triisopropylsilyl)acetylene)benzamide (3b)
[0113]
[0114] Under nitrogen atmosphere, arylamide compound 1b (16.4 mg, 0.10 mmol), alkynyl bromide or alkyne 2 (30 μL, 0.15 mmol), dichloro(pentamethylcyclopentadienyl ) iridium dimer (2.3mg, 0.0025mmol) or dichloro(p-methylcumene) ruthenium dimer (1.5mg, 0.0025mmol), bis(trifluoromethanesulfonyl)imide silver (4.2 mg, 0.015mmol) or silver hexafluoroantimonate (5.2mg, 0.015mmol), cesium acetate (30mg, 0.36mmol), (when the reaction uses an alkyne bromide, no additional oxidant is needed; when the reaction uses a terminal alkyne directly, then Need 2 equivalents of silver acetate), 1,2-dichloroethane (DCE, 1mL), react at 120°C for 12 hours. After the reaction was completed, it was cooled to room temperature, filtered through diatomaceous earth, and concentrated to obtain a crude product. The crude product was chromatographically separated on a prepared silica gel plate, and the se...
Embodiment 3
[0118] Example 3 3-((triisopropylsilyl)acetylene)isonicotinamide (3c)
[0119]
[0120] Under nitrogen atmosphere, add arylamide compound 1c (12.2 mg, 0.10 mmol), alkyne 2 (40 μL, 0.40 mmol), dichloro(pentamethylcyclopentadienyl) iridium di polymer (2.3mg, 0.0025mmol) or dichloro(p-methylcymene) ruthenium dimer (1.5mg, 0.0025mmol), silver bis(trifluoromethanesulfonyl)imide (4.2mg, 0.015mmol ) or silver hexafluoroantimonate (5.2mg, 0.015mmol), cesium acetate (30mg, 0.36mmol), (when the reaction uses alkyne bromide, no additional oxidant is needed; when the reaction directly uses terminal alkynes, 2 equivalents of Silver acetate), 1,2-dichloroethane (DCE, 1mL), react at 120°C for 16 hours. After the reaction was completed, cool to room temperature, filter through diatomaceous earth, and concentrate the crude product obtained by chromatography on a prepared silica gel plate. The selected developer was petroleum ether and ethyl acetate with a volume ratio of 3:1. The product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com