Kluyveromyces marxianus aldehyde ketone reductase KmAKR mutant and application thereof
A technology of yeast aldehydes, ketones, and reductases, which is applied in the direction of oxidoreductases, fermentation, etc., can solve the problems of low asymmetric reduction activity and low substrate feeding amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Construction and screening of aldehyde and ketone reductase mutant library
[0036] The Kluyveromyces marx aldoketone reductase mutant library was prepared by one round of site-directed saturation mutagenesis. The primers were designed as shown in Table 1. E.coli BL21(DE3) / pET28a(+)-kmakr-W297H / Y296W / K29H / Y28A (see patent application CN201910072740.9 for construction) in the vector pET28a(+)-kmakr-W297H / Y296W / K29H / Y28A as a template, using Thr63-F and Thr63-R in Table 1 as primers, through saturation mutation PCR, the The 63rd threonine of the aldehyde and ketone reductase KmAKR-W297H / Y296W / K29H / Y28A amino acid sequence shown in SEQ ID NO.2 was mutated into the remaining 19 kinds of amino acids, and transformed, plated, and screened by dominant strains (Table 2) , to obtain the aldehyde and ketone reductase mutant KmAKR-W297H / Y296W / K29H / Y28A / T63A (denoted as M5-A, that is, the 63rd threonine of the amino acid shown in SEQ ID NO.2 is mutated to alanine), KmAK...
Embodiment 2
[0040] Embodiment 2: Induced expression of control group aldehyde and ketone reductase, mutant and glucose dehydrogenase
[0041] Glucose dehydrogenase genetically engineered bacteria: insert the glucose dehydrogenase gene (GenBank NO.KM817194.1) from E.sibirium DSM 17290 into pET28b(+) to construct a recombinant expression vector, and transfer the expression vector into E.coli BL21 (DE3) E. coli BL21(DE3) / pET28b(+)-esgdh was produced.
[0042] The starting strain E.coli BL21(DE3) / pET28a(+)-kmakr-W297H / Y296W / K29H / Y28A from Example 1 and the aldehyde and ketone reductase mutant strain screened in Example 1 and E.coli BL21(DE3) / pET28b (+)-esgdh were respectively inoculated into LB liquid medium containing a final concentration of 50 μg / mL kanamycin, cultured at 37°C for 10 h, and inoculated into fresh LB medium containing a final concentration of 1.5% (v / v) 50μg / mL kanamycin LB liquid medium, cultured at 37°C, 180rpm for 2h, then added 0.15mM IPTG to the culture solution, cultu...
Embodiment 3
[0043] Example 3: Mutant library screening
[0044]Mix the wet cells of the mutant strain induced and expressed in Example 2 and the wet cells of glucose dehydrogenase at a dry weight ratio of 3.5:1 (w / w) to form a mixed cell, add pH 7.0, 100mM PBS buffer to resuspend, Obtain the mixed bacterial liquid of mutant strains. Under the same conditions, the control strain E.coli BL21(DE3) / pET28a(+)-kmakr-W297H / Y296W / K29H / Y28A was used to replace the wet cells of the mutant strain to prepare a mixed bacterial solution of the control strain.
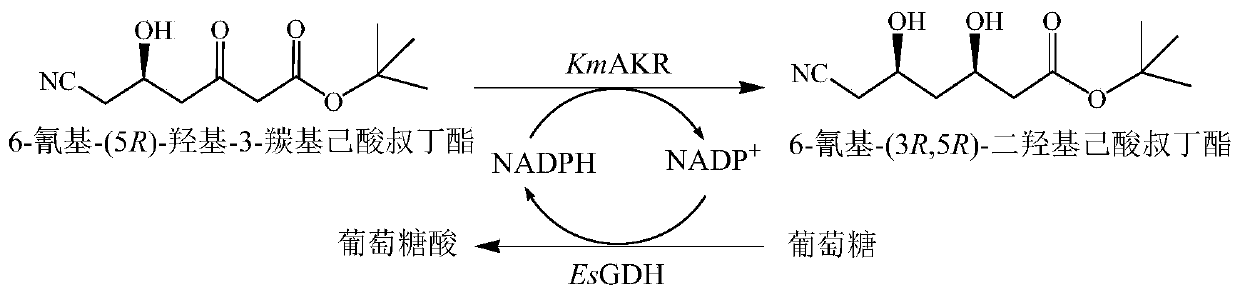
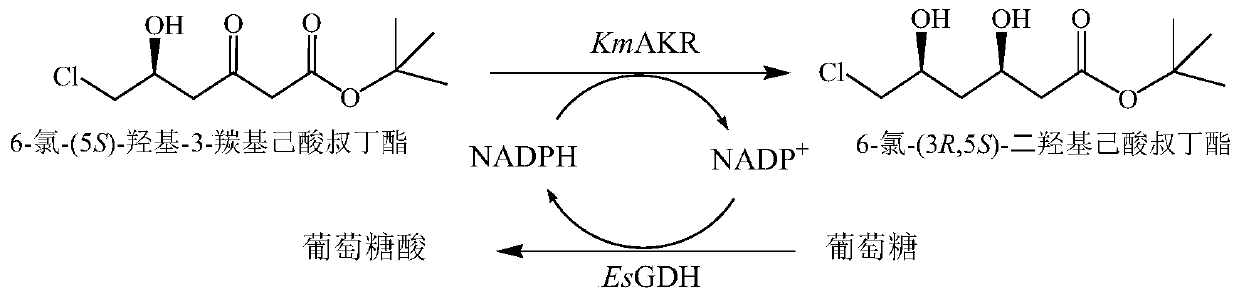
[0045] The mixed bacterial solution of the mutant strain and the mixed bacterial solution of the control strain were used as catalysts, tert-butyl 6-cyano-(5R)-hydroxy-3-carbonylhexanoate was used as the substrate, glucose was used as the auxiliary substrate, and no external derived NADPH or NADP + , using the endogenous NADPH of bacterial cells to establish a coenzyme circulation system. The reaction system is selected as 10mL, the amount of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com