A precious metal alloy core-shell catalyst prepared by using an organic reducing agent and its preparation method
A noble metal and catalyst technology, applied in the field of high-performance ultrafine low-Pt core-shell catalyst and its preparation, can solve the problems of high cost and insufficient lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
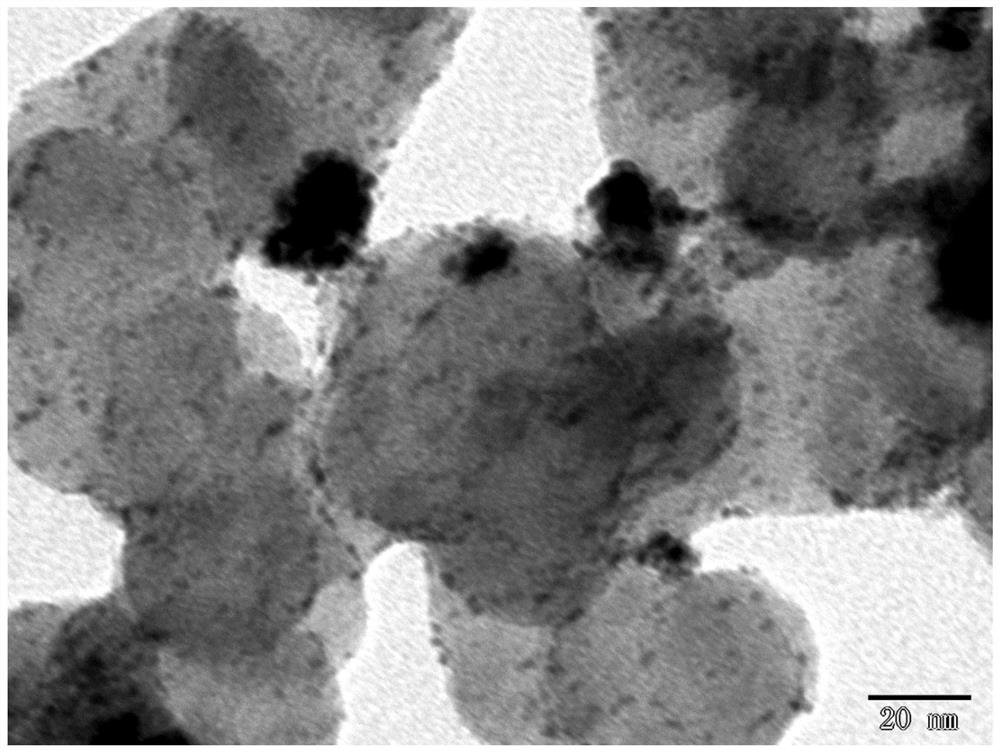
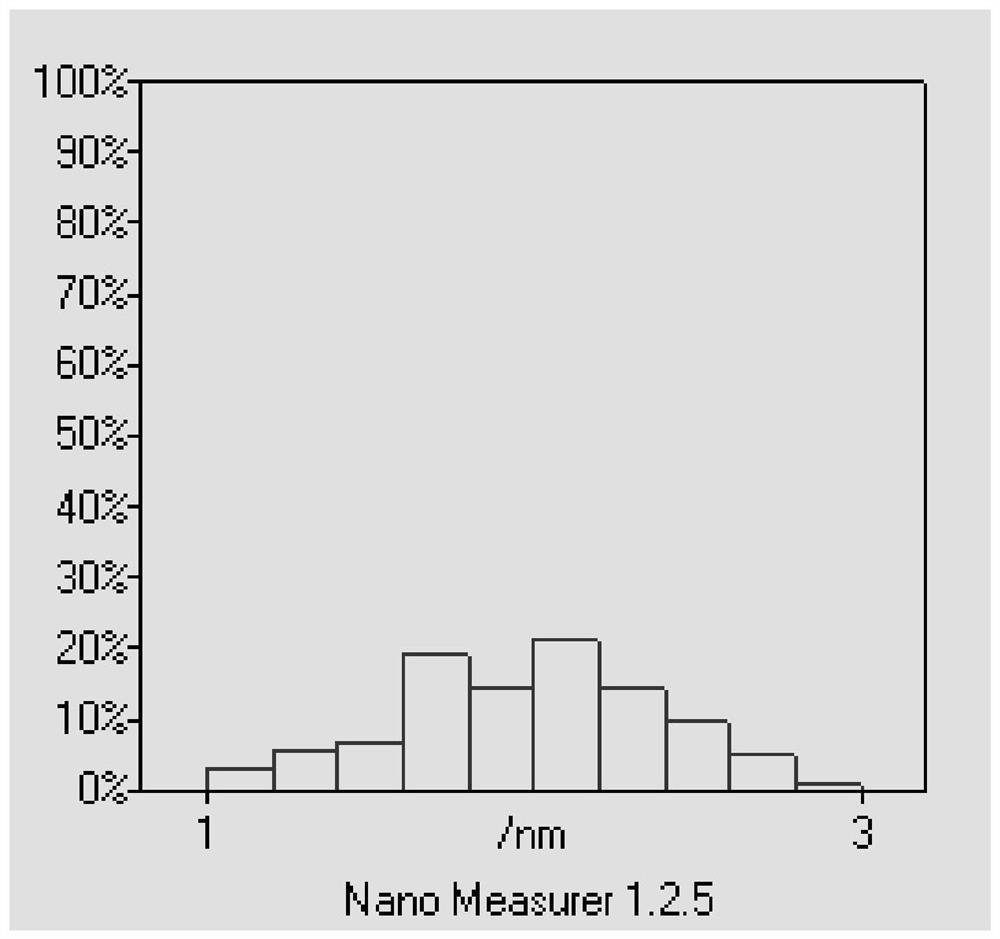
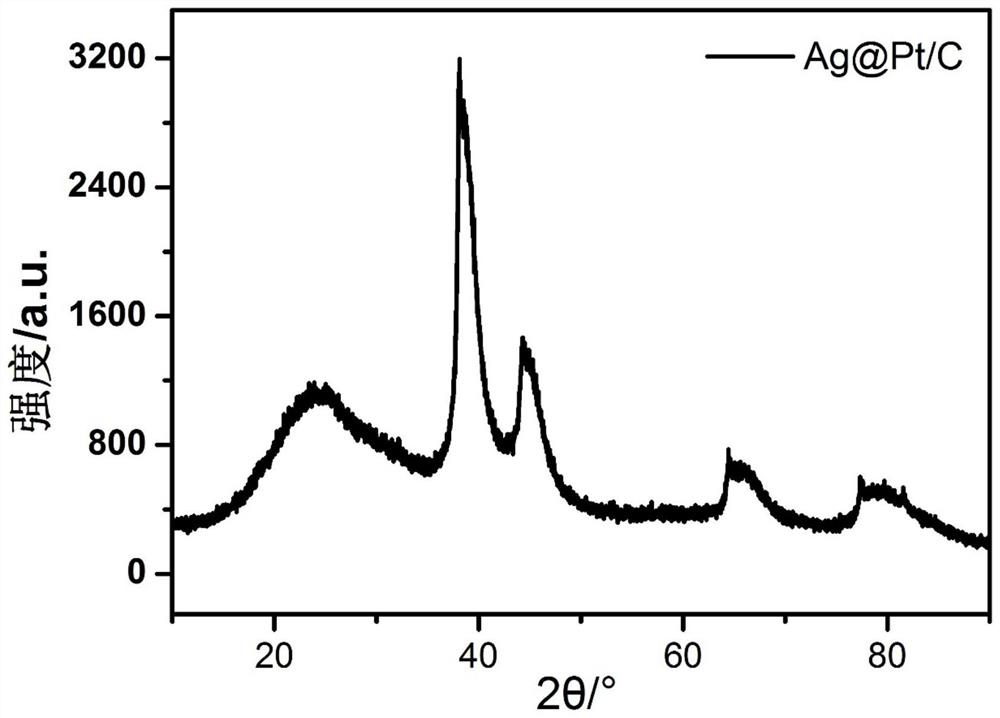
[0057] First, 1wt.% AgNO 3 Dilute the solution in 100mL of deionized water, add 0.034g of protective surfactant CTAB and 50mg of loaded carbon carrier XC-72, ultrasonically disperse for 30min, then heat the oil bath to 105°C, add 1wt.% sodium citrate 10mL, carry out Reduction of carboxyl and hydroxyl groups, oil bath reduction temperature 105°C, time 1h; cool down to 60°C, add 0.015mol / L H 2 PtCl 6 3mL, replace at 60°C for 2h to prepare a transition layer; mix 1.2g of ascorbic acid with H 2 PtCl 6 3mL, dissolve and mix with 25mL and add, adjust pH=10 with NaOH, carry out carboxyl and hydroxyl reduction at 60°C for 4h, finally add 0.2gNaBH 4 Mix solution with 0.06g NaOH, stir and age overnight; centrifuge and dry to obtain Ag@Pt / C (60°C) catalyst. The physical characterization of the catalyst is shown in Figures 1-3, and the performance of the catalyst is shown in Figure 4.
[0058] Figure 1 is a low magnification TEM image of the catalyst prepared in Example 1, (a) it c...
Embodiment 2
[0063] First, 1wt.% AgNO 3 Dilute the solution in 100mL of deionized water, add 0.034g of protective surfactant CTAB and 50mg of loaded carbon carrier XC-72, ultrasonically disperse for 30min, then heat the oil bath to 105°C, add 1wt.% sodium citrate 10mL, carry out Reduction of carboxyl and hydroxyl groups, oil bath reduction temperature 105°C, time 1h; maintain 105°C, add 0.015mol / L H2PtCl6 3mL, replace at 105°C for 2h, prepare transition layer; ascorbic acid 1.2g, H 2 PtCl 6 3mL, dissolve and mix with 25mL and add, adjust pH=10 with NaOH, carry out carboxyl and hydroxyl reduction at 105°C for 4h, finally add 0.2gNaBH 4 Mix solution with 0.06g NaOH, stir and age overnight; centrifuge and dry to obtain Ag@Pt / C (60°C) catalyst. Catalyst performance is shown in Figure 5.
[0064] Fig. 5 is a graph showing the electrochemical performance of the catalyst prepared in Example 2 of the present invention. (a) Comparison of CV activity between prepared Ag@Pt / C catalyst and commer...
Embodiment 3
[0066]Electrochemical tests were performed in a three-electrode system to characterize the oxygen reduction activity of the catalysts. The electrolyte solution of this system is 0.1mol L -1 HClO 4 , the counter electrode is a Pt sheet electrode, the reference electrode is a saturated calomel electrode, the cyclic voltammetry test electrolyte solution is saturated with N2, and the test system is Gamry3000; the ORR test solution is saturated with O2. Preparation of the catalytic layer of the rotating disk electrode membrane: 40% Pt / C catalyst: 5mg catalyst, 2.5mL isopropanol, ultrasonication; add 50 μL of 5wt% Nafion solution, ultrasonication, take 3.2 μL of the above dispersed slurry and coat it on a rotating disk electrode The disc electrode surface, as the working electrode. Due to the low loading of the Ag@Pt / C catalyst, preparation of the membrane catalyst layer: 5mg catalyst, 2.5mL isopropanol, ultrasonication; add 50μL of 5wt% Nafion solution, ultrasonication, take 6.4μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com