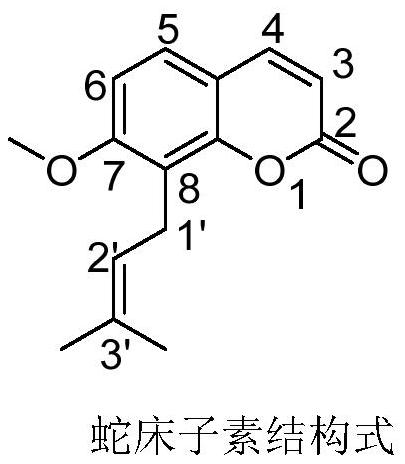
Osthole ester derivatives, preparation method and application thereof
A technology of osthole and its derivatives, which is applied in the direction of botany equipment and methods, applications, biocides, etc., can solve the problems of general activity and narrow spectrum of insecticidal action, and achieve simple preparation process, high yield, and excellent killing effect. The effect of insect activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthesis of embodiment 1 osthole ester derivatives
[0037] (1) Synthesis of Thioosthole (b)
[0038] Dissolve 486mg of osthole and 809mg of Lawson's reagent in anhydrous tetrahydrofuran, heat to reflux at 66°C, and detect by TLC. After 24 hours, the reaction is completed. The reaction solution is concentrated under reduced pressure and separated by column chromatography to obtain 351mg of thiocnidium. prime (b).
[0039] Physicochemical properties of compound (b):
[0040] 1), yellow solid, melting point 118-120°C;
[0041] 2), the infrared spectrogram feature (IR) feature of compound (b):
[0042] Using potassium bromide tablet method: 2964, 2927cm -1 Stretching vibration absorption for saturated hydrocarbons, 1597, 1549, 1504cm -1 For the absorption of aromatic ring stretching vibration, 1130, 1255cm -1 It is C-O-C stretching vibration absorption.
[0043] 3), the nuclear magnetic resonance spectrum characteristic of compound (b): 1 H NMR (400MHz, CDCl ...
Embodiment 2
[0059] The synthesis (compound 2) of embodiment 2 osthole ester derivatives
[0060] Using the method described in Example 1, compound (c) reacts with 3-bromobenzoic acid to synthesize compound 2. The structure and physicochemical properties of compound 2 are as follows:
[0061]
[0062] 1), pale yellow solid, melting point 140-142°C;
[0063] 2), the infrared spectrogram feature (IR) feature of this compound:
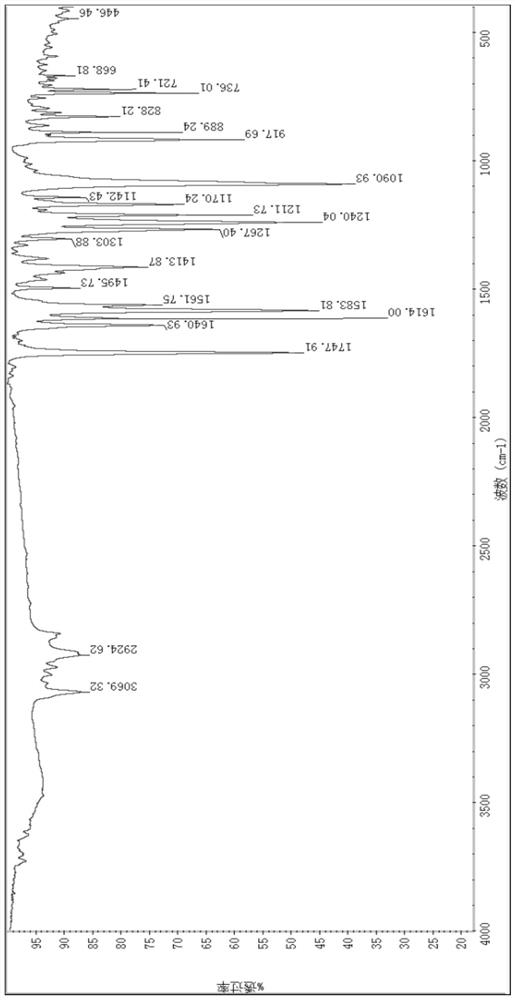
[0064] Using potassium bromide tablet method: 3069cm -1 It is unsaturated hydrocarbon stretching vibration absorption, 2924cm -1 Stretching vibration absorption for saturated hydrocarbons, 1747cm -1 Absorbing stretching vibration for ester carbonyl group, 1583cm -1 It is the vibration absorption of aromatic ring C-C skeleton, 1240, 1090cm -1 It is C-O-C stretching vibration absorption.
[0065] 3), the nuclear magnetic resonance spectrum characteristic of this compound: 1 H NMR (400MHz, CDCl 3 )δ:8.33(s,1H,-Ar),8.11(d,J=8.0Hz,1H,-Ar),7.70-7.73(m,1H,-Ar),7.3...
Embodiment 3
[0066]Synthesis of embodiment 3 osthole ester derivatives (compound 3)
[0067] Using the method described in Example 1, compound (c) reacts with 2-methoxybenzoic acid to synthesize compound 3. The structure and physicochemical properties of compound 3 are as follows:
[0068]
[0069] 1), white solid, melting point 150-152°C;
[0070] 2), the infrared spectrogram feature (IR) feature of this compound:
[0071] Using potassium bromide tablet method: 3075, 3004cm -1 Stretching vibration absorption for unsaturated hydrocarbons, 2928, 2838cm -1 Stretching vibration absorption for saturated hydrocarbons, 1735cm -1 Absorbing stretching vibration for ester carbonyl group, 1582cm -1 It is the vibration absorption of aromatic ring C-C skeleton, 1259,1063cm -1 It is C-O-C stretching vibration absorption.
[0072] 3), the nuclear magnetic resonance spectrum characteristic of this compound: 1 H NMR (400MHz, CDCl 3 )δ: 8.14(d,J=8.8Hz,2H,-Ar),7.09-7.14(m,2H,-Ar),6.92(d,J=8.8Hz,2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com