Trinuclear copper alkynyl group complex with amino acid recognition function and preparation method of trinuclear copper alkynyl group complex
An amino acid and complex technology, applied in the field of amino acid recognition, can solve the problems of rare research on ligand carbonyl-containing complexes, cumbersome pretreatment steps, etc., and achieve the effects of safe and reliable reaction process, high yield and simple preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
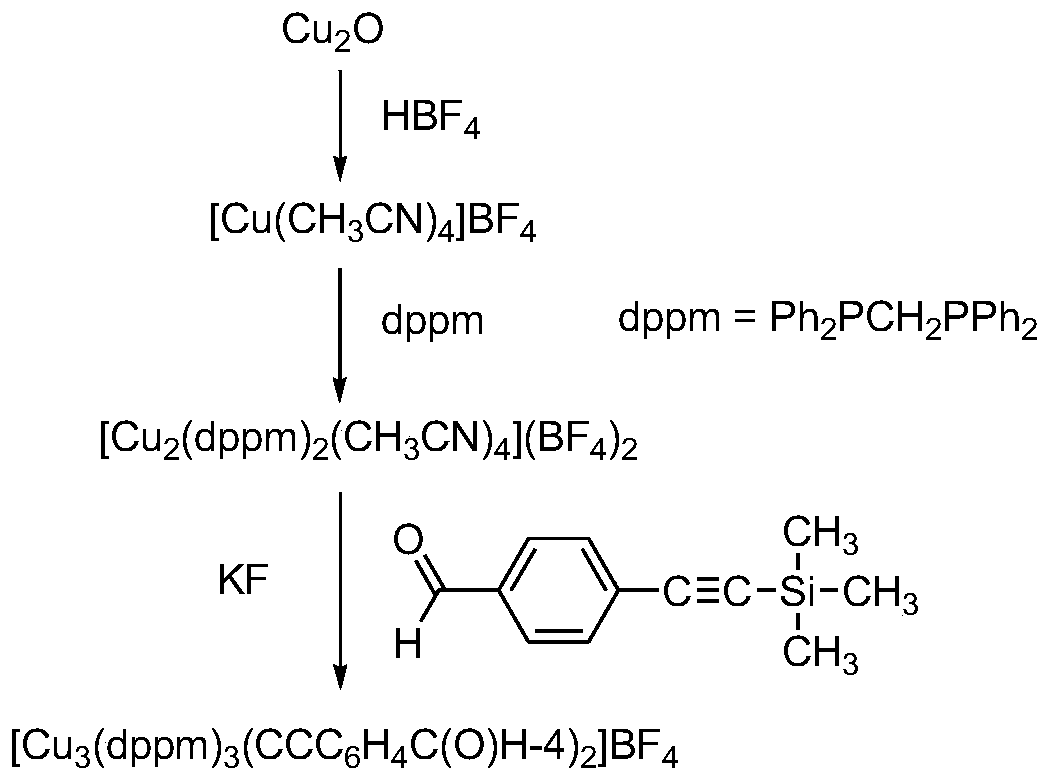
[0048] [Cu(CH 3 EN) 4 ](BF 4 )Synthesis:
[0049] 30.3mmol Cu 2 O was dissolved in 40 mL of acetonitrile, and 10 mL of HBF was slowly added dropwise 4 to red Cu 2 O completely disappeared, continue to add acetonitrile until the precipitate disappears, remove the unreacted solid by suction filtration, remove the solvent under reduced pressure, and obtain a white solid after adding ether.
[0050] [Cu 2 (dppm) 2 (CH 3 EN) 4 ](BF 4 ) 2 Synthesis:
[0051] 8mmol[Cu(CH 3 EN) 4 ](BF 4 ), 8mmol of bis(diphenylphosphine)methane, and 40mL of acetonitrile were added to the flask, and reacted for 24 hours under nitrogen protection. Part of the solvent was removed, and ether was added as a white solid (yield 81%).
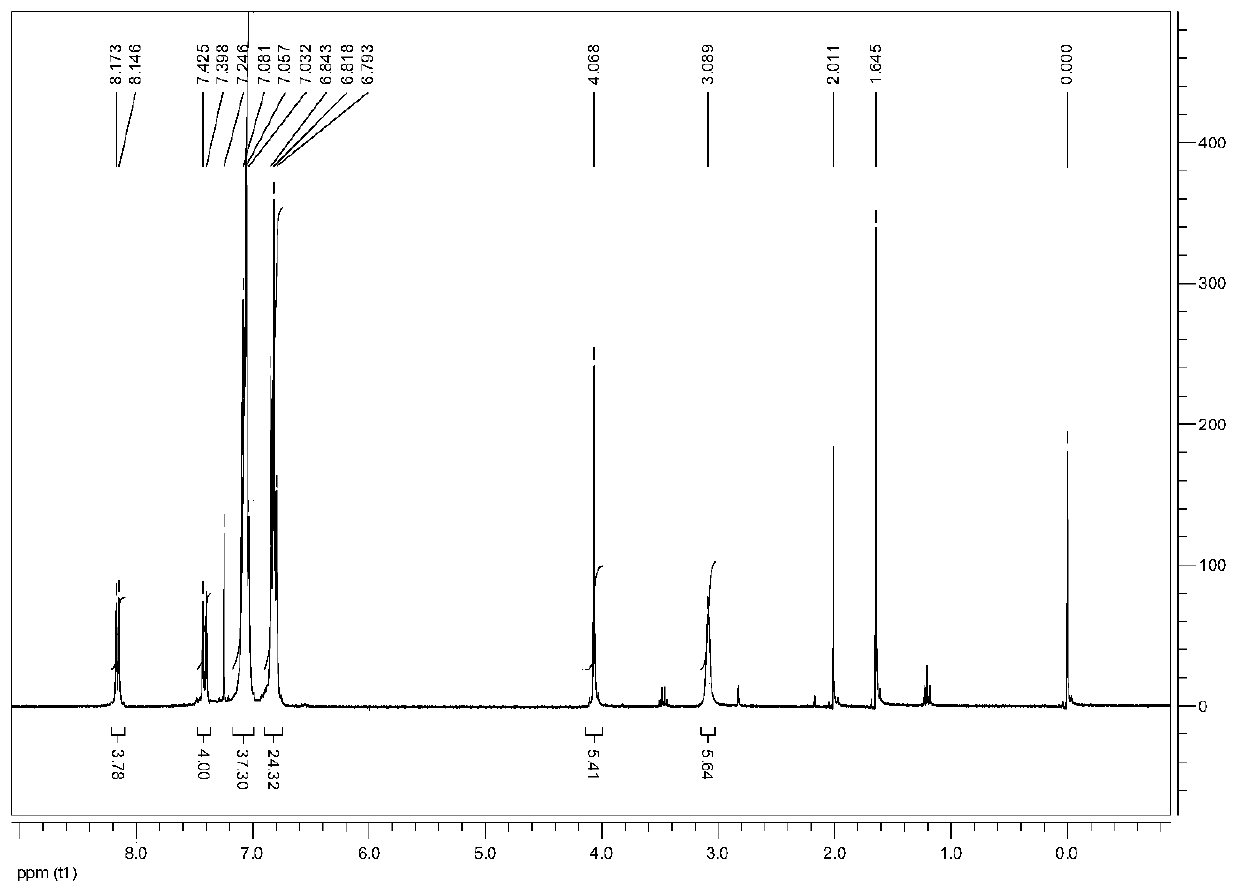
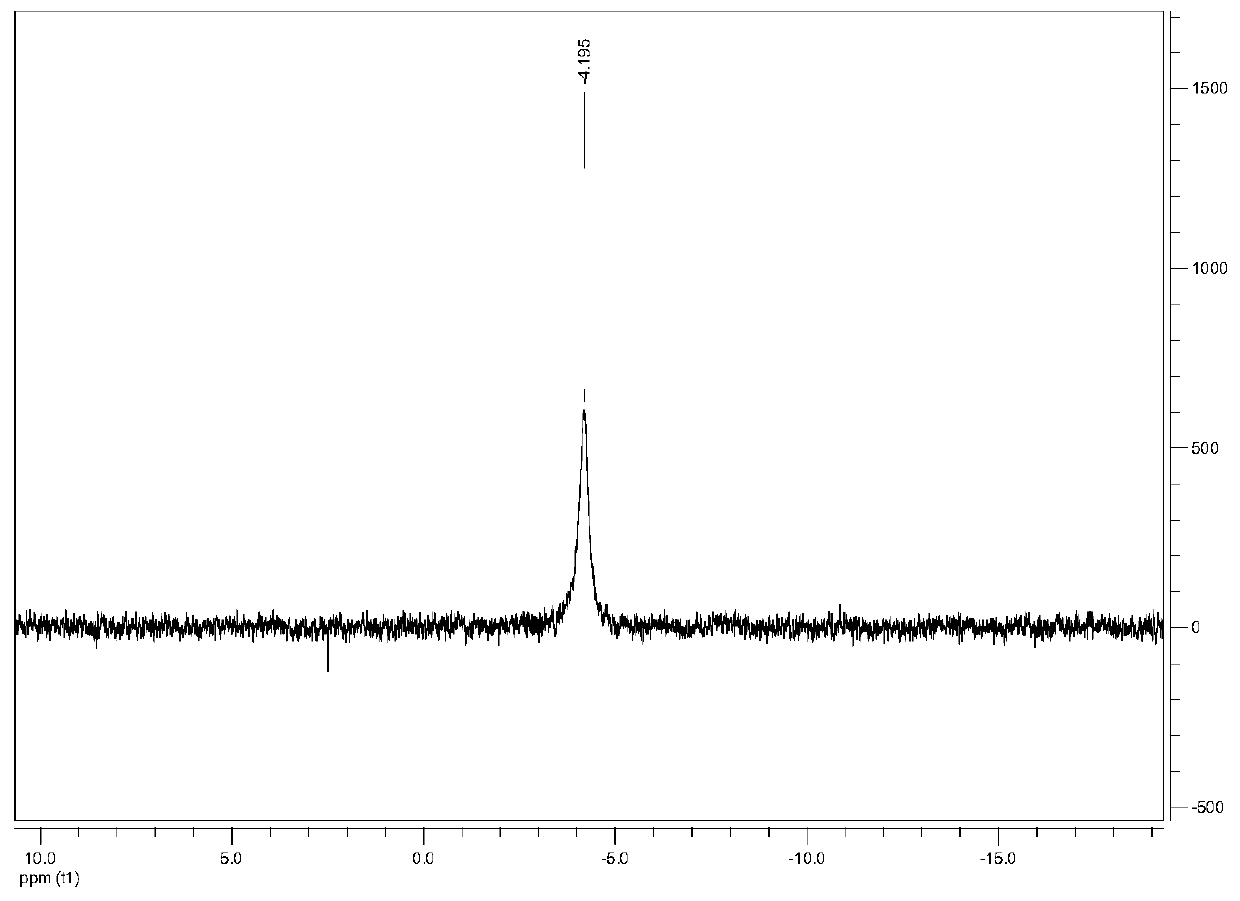
[0052] [Cu 3 (μ-dppm) 3 (μ 3 -η 1 -C≡CC 6 h 4 -C(O)H-4) 2 ](BF 4 )Synthesis:
[0053] To 0.12mmol[Cu] dissolved in 20mL acetonitrile 2 (dppm) 2 (CH 3 EN) 4 ](BF 4 ) 2 , 0.15mmol 4-trimethylsilylethynyl benzaldehyde, add 0.24mmol KF·2H dissolved in...
Embodiment 2
[0057] [Cu(CH 3 EN) 4 ](BF 4 )Synthesis:
[0058] 30.3mmol Cu 2 O was dissolved in 40 mL of acetonitrile, and 10 mL of HBF was slowly added dropwise 4 to red Cu 2 O completely disappeared, continue to add acetonitrile until the precipitate disappears, remove the unreacted solid by suction filtration, remove the solvent under reduced pressure, and obtain a white solid after adding ether.
[0059] [Cu 2 (dppm) 2 (CH 3 EN) 4 ](BF 4 ) 2 Synthesis:
[0060] 8mmol[Cu(CH 3 EN) 4 ](BF 4 ), 8mmol of bis(diphenylphosphine)methane, and 40mL of acetonitrile were added to the flask, and reacted for 24 hours under nitrogen protection. Part of the solvent was removed, and ether was added as a white solid (yield 81%).
[0061] [Cu 3 (μ-dppm) 3 (μ 3 -η 1 -C≡CC 6 h 4 -C(O)H-4) 2 ](BF 4 )Synthesis:
[0062] To 0.12mmol[Cu] dissolved in 20mL acetonitrile 2 (dppm) 2 (CH 3 EN) 4 ](BF 4 ) 2 , 0.15mmol 4-trimethylsilylethynyl benzaldehyde, add 0.24mmol KF·2H dissolved in...
Embodiment 3
[0066] UV-Vis absorption spectrometry titration detection:
[0067] [Cu prepared in Example 1] 3 (μ-dppm) 3 (μ 3 -η 1 -C≡CC 6 h 4 -C(O)H-4) 2 ](BF 4 ) 10μmol / L, and add cysteine, carry out ultraviolet-visible absorption spectrum titration, the result is as attached Figure 4 As shown, it can be concluded that the binding constant of the trinuclear copper alkynyl complex prepared in Example 1 and cysteine is lg K=4.41±0.05.
[0068] [Cu prepared in Example 1] 3 (μ-dppm) 3 (μ 3 -η 1 -C≡CC 6 h 4 -C(O)H-4) 2 ](BF 4 ) 10μmol / L, and add homocysteine, carry out ultraviolet-visible absorption spectrum titration, the result is as attached Figure 5 As shown, it can be concluded that the binding constant of the trinuclear copper alkynyl complex prepared in Example 1 and homocysteine is lg K=4.84±0.11.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com