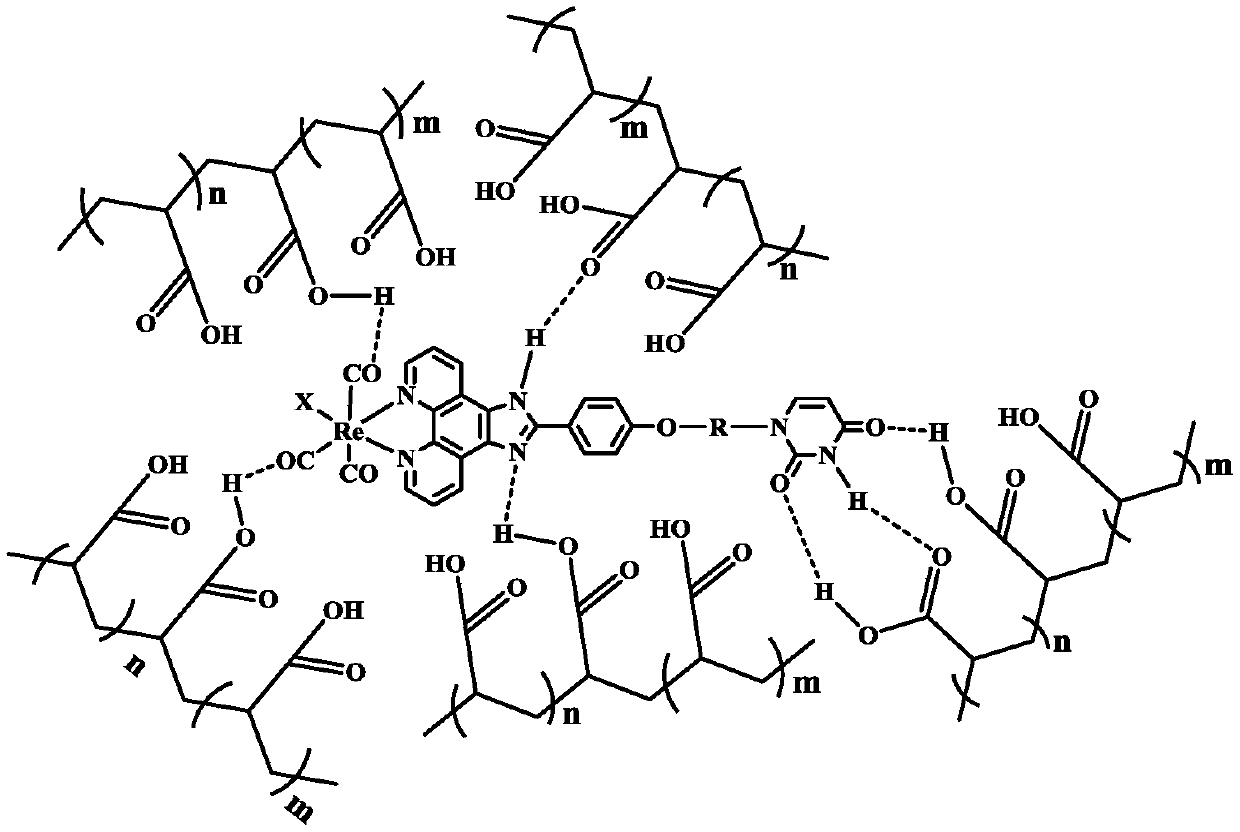
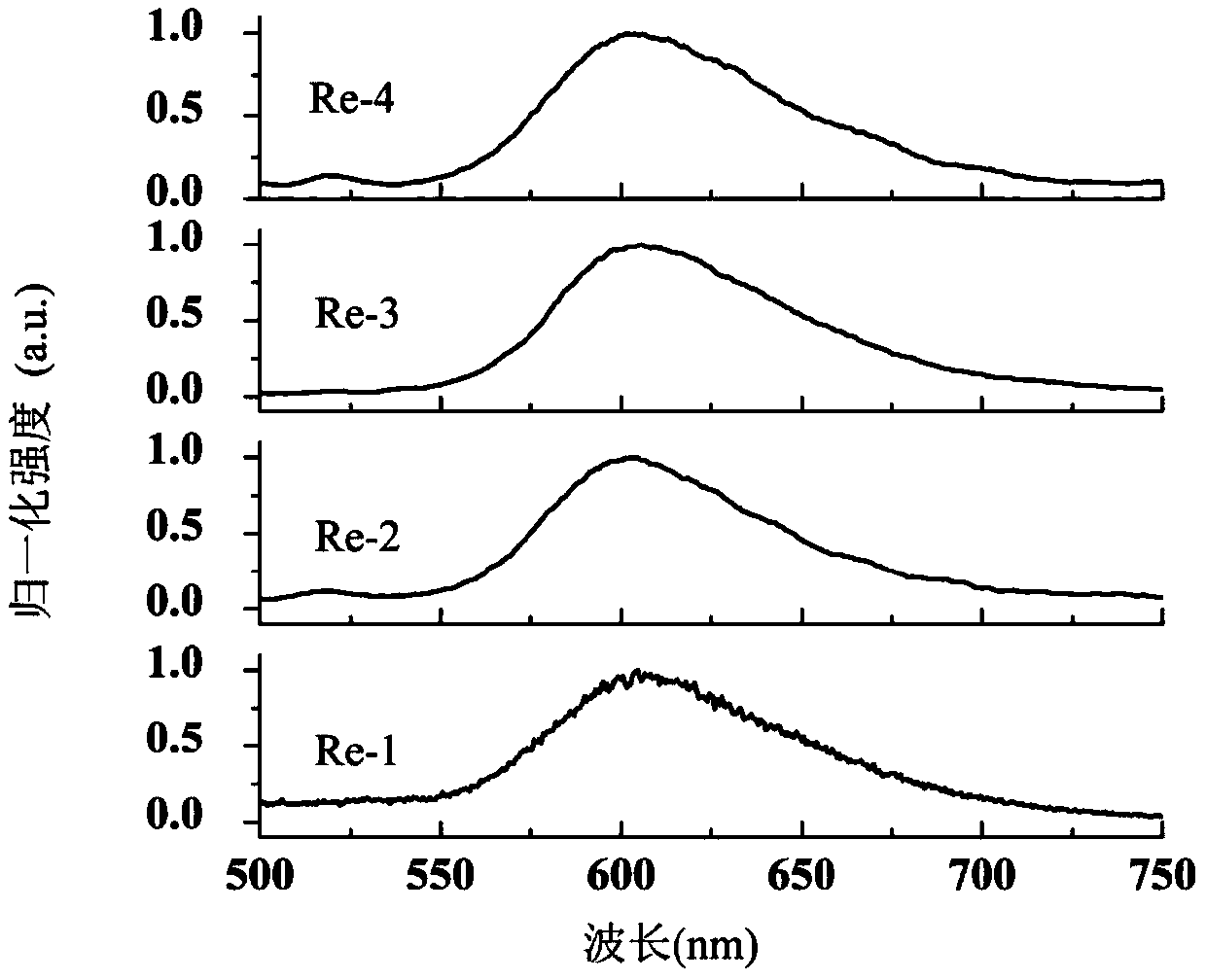
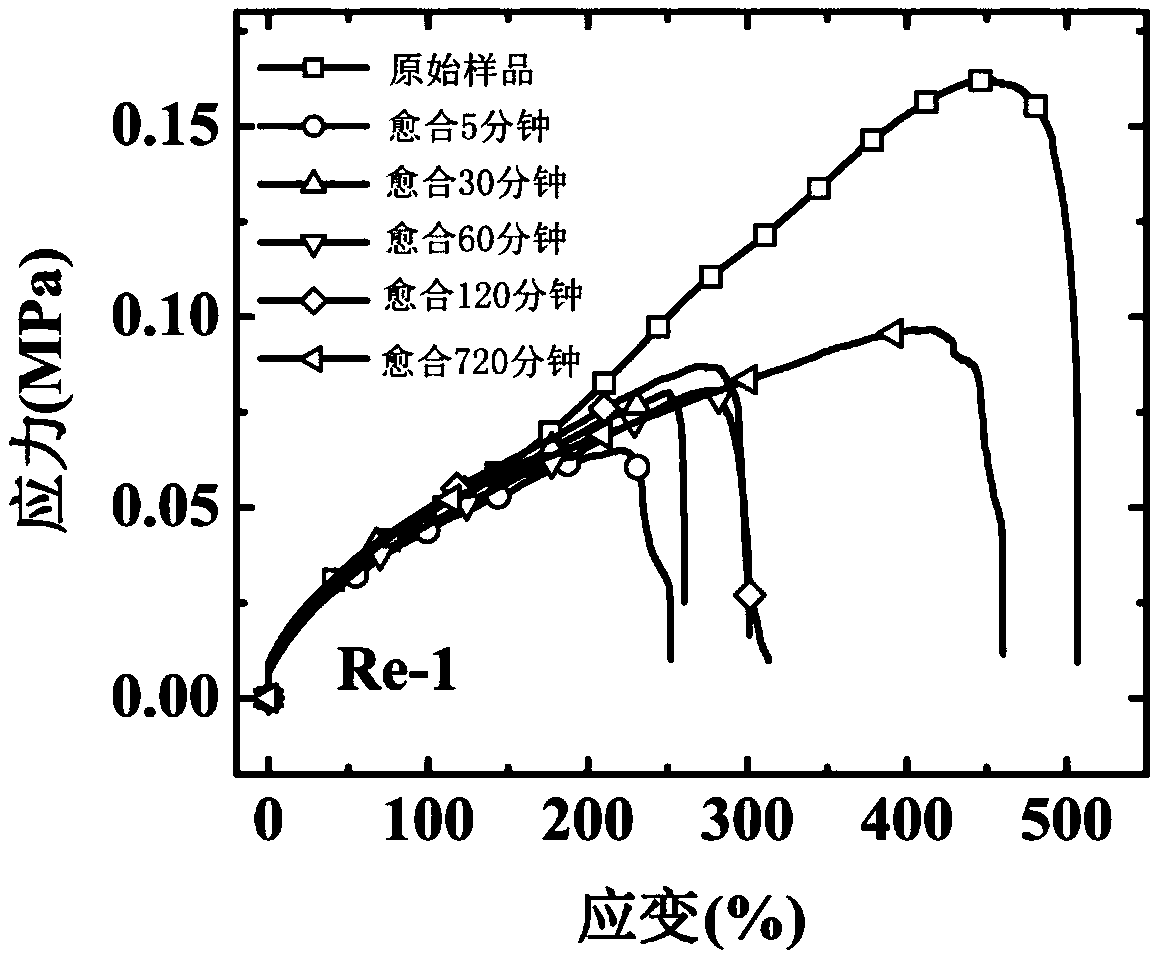
Method for preparing self-healed phosphorescence organic gel material
An organic gel, self-healing technology, applied in the direction of luminescent materials, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the synthesis of diimine ligand Ura-Phen-1 in structural formula (I)
[0024]
[0025] Under nitrogen protection, 4-(4-bromobutoxy)benzaldehyde (1.028g, 4mmol), uracil (0.672g, 6mmol) and anhydrous potassium carbonate (0.828g, 6mmol) were successively added to 30mL N, In N-dimethylformamide, the reaction was continued for 36 hours after slowly heating to 80°C. After the temperature dropped to room temperature, 150 mL of deionized water was added to the reaction system. Stir at room temperature until the solution is clear and then filter. The collected crude product is purified by column chromatography using ethyl acetate / petroleum ether (v / v=2 / 1) as eluent to obtain 0.644 g of white Ura-CHO-1 solid (56% yield). Ura-CHO-1 (0.576 g, 2 mmol), 1,10-phenanthroline-5,6-dione (0.420 g, 2 mmol) and ammonium acetate (1.540 g, 20 mmol) were added to 15 mL of glacial acetic acid, The reaction was continued for 6 hours after heating to 118° C. under the protecti...
Embodiment 2
[0026] Embodiment 2: the synthesis of diimine ligand Ura-Phen-2 in structural formula (I)
[0027]
[0028] The synthesis method of Ura-Phen-2 is similar to that of Ura-Phen-1, replacing 4-(4-bromobutoxy)benzaldehyde (1.028g, 4mmol) with 4-(6-bromohexyloxy)benzaldehyde (1.140 g, 4 mmol)), and 0.732 g of white Ura-CHO-2 solid powder was obtained (58% yield). Then Ura-CHO-2 (0.101 g, 2 mmol), 1,10-phenanthroline-5,6-dione (0.420 g, 2 mmol) and ammonium acetate (1.540 g, 20 mmol) were added to 15 mL of glacial acetic acid, Heated to 118°C under nitrogen protection and continued to react for 6 hours. After the temperature dropped to room temperature, 150 mL of deionized water was added to the reaction system, and the pH of the reaction system was adjusted to about 7 with 10% aqueous sodium hydroxide solution. The precipitated solid was recrystallized from methanol to collect 0.138 g of pale yellow Ura-Phen-2 solid powder (81% yield). 1 H NMR (DMSO, 400Hz, ppm): δ = 1.350 (t,...
Embodiment 3
[0029] Embodiment 3: the synthesis of diimine ligand Ura-Phen-3 in structural formula (I)
[0030]
[0031] The synthesis method of CHO-Ura-3 is similar to that of CHO-Ura-1, replacing 4-(4-bromobutoxy)benzaldehyde (1.028g, 4mmol) with 4-(8-bromooctyloxy)benzaldehyde (1.252g, 4mmol), 0.728g of white CHO-Ura-3 solid powder was obtained, the yield was 53%. CHO-Ura-3 (0.688g, 2mmol), 1,10-phenanthroline-5,6-dione (0.420g, 2mmol) and ammonium acetate (1.540g, 20mmol) were added to 15mL of glacial acetic acid, in Heated to 118°C under nitrogen protection and continued to react for 6 hours. After the temperature dropped to room temperature, 150 mL of deionized water was added to the reaction system, and the pH of the reaction system was adjusted to about 7 with 10% aqueous sodium hydroxide solution. After the precipitated solid was recrystallized from methanol, 0.982 g of light yellow Ura-Phen-3 solid powder was collected (89% yield). 1 H NMR (DMSO, 400Hz, ppm): δ = 1.276 (d, ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap