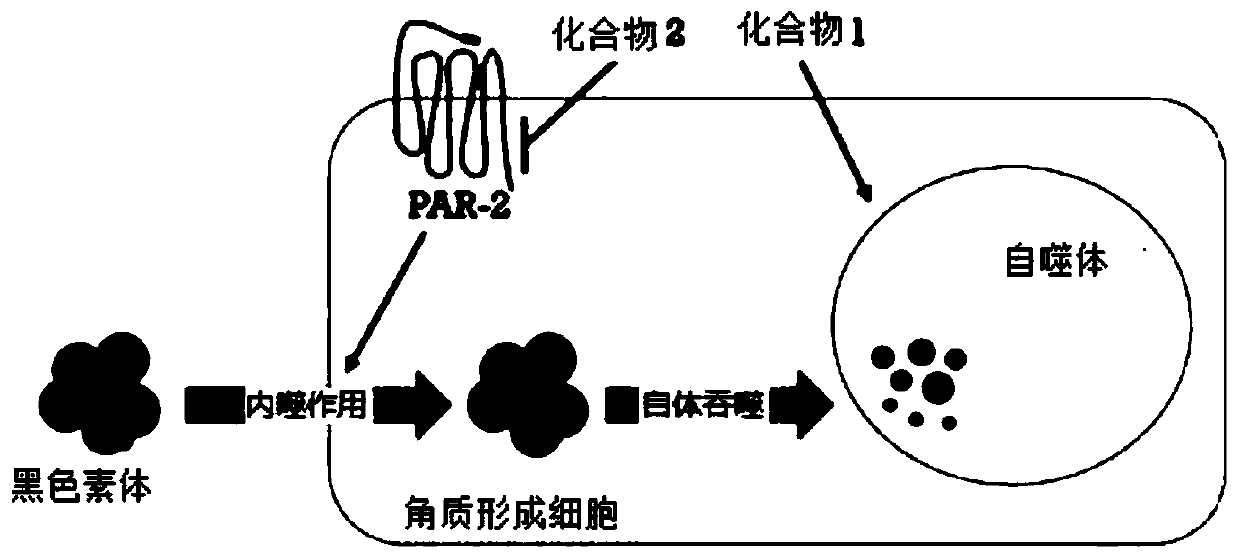
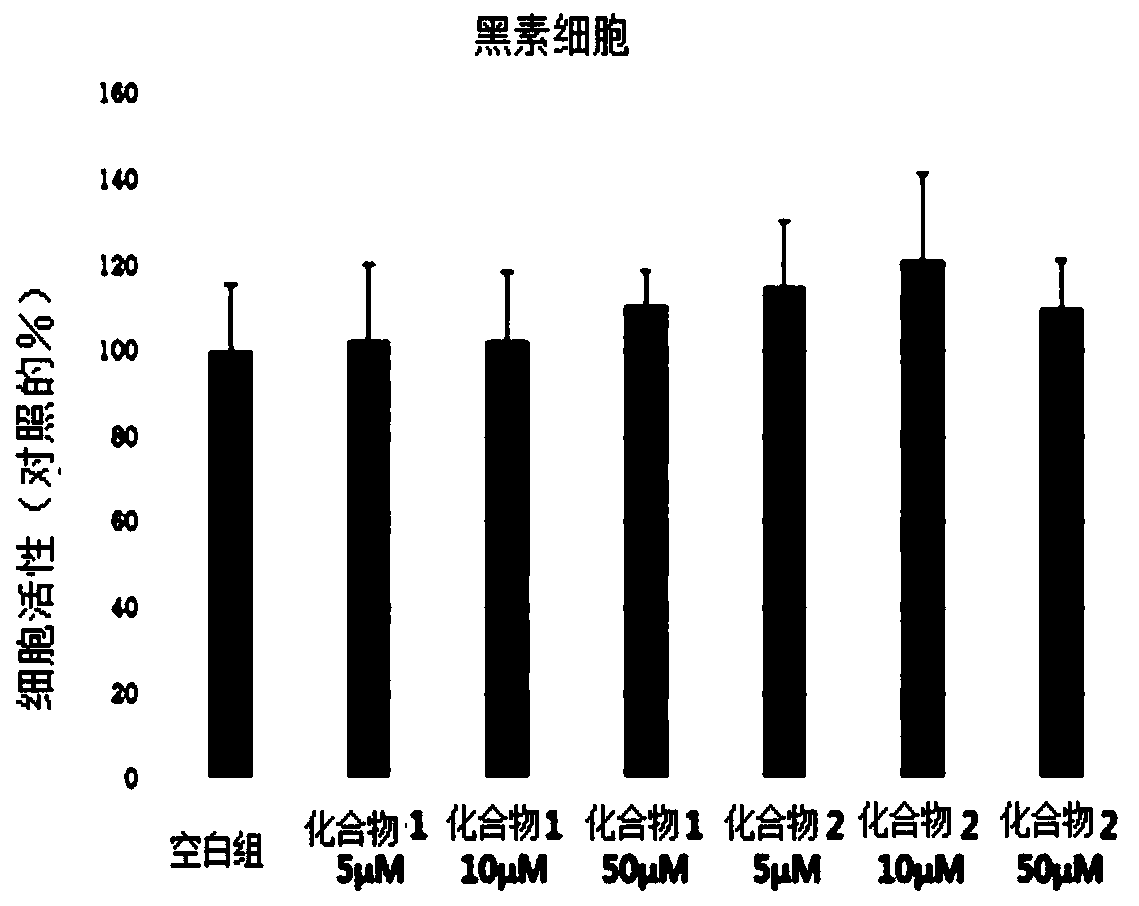
Novel compound, and cosmetic composition and pharmaceutical composition comprising the same
A technology of compound and composition, applied in the direction of pharmaceutical combination, cosmetics, organic chemistry, etc., can solve the problems of hyperpigmentation, unevenness, etc., achieve the effect of reducing melanin pigmentation, reducing melanin production, and preventive treatment of hyperpigmentation diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Embodiment 1. Synthesis of compound 1
[0147] Step 1. Compound 1a: Synthesis of Fmoc-Lys(Dde)-O-2-chlorotrityl resin (Fmoc-Lys(Dde)-O-2-chloro trityl resin)
[0148]
[0149] Make 2-chlorotrityl chloride resin (2-chloro trityl chloride resin, 100-200 mesh (mesh), Novabiochem 100mg, 1 equivalent) and Fmoc-Lys(Dde)-OH (Nα-Fmoc-Nε-Dde- L-lysine, Nα-Fmoc-Nε-[1-(4,4-dimethyl-2,6-dioxocyclohexylidene) ethyl]-L-lysine (Fmoc-Lys( Dde)-OH(Nα-Fmoc-Nε-Dde-L-lysine, Nα-Fmoc-Nε-[1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethyl]-L-lysine), 106.5mg, 2 equivalents) and diisopropylamine (DIPEA, 69.7 μL, 4 equivalents) were dissolved in 5 ml of dichloromethane (DCM) and added to a 10 ml reaction vessel, and reacted at normal temperature (23° C.) for 12 hours. The reaction solution was removed by filtration, and the synthesized resin was sequentially washed with 10 ml of dichloromethane, methanol (MeOH), dichloromethane, and dimethylformamide (DMF), respectively, thereby quantitativel...
Embodiment 2
[0163] Embodiment 2: the synthesis of compound 2
[0164] Step 1. Compound 2a: Synthesis of Fmoc-Hyp(tBu)-O-2-chlorotrityl resin (Fmoc-Hyp(tBu)-O-2-chloro trityl resin)
[0165]
[0166] Make 2-chlorotrityl chloride resin (100-200 mesh, Novabiochem 200mg, 1 equivalent) and Fmoc-Hyp(tBu)-OH(Fmoc-O-tert-butyl-L-hydroxyproline) (Fmoc- Hyp(tBu)-OH(Fmoc-O-tert-butyl-L-hydroxyproline), 163.8mg, 2 equivalents) and DIPEA (139.3ul, 4 equivalents) were dissolved in 5ml of dichloromethane and added to a 10ml reaction vessel , and reacted at normal temperature for 12 hours. The reaction solution was removed by filtration, and the synthesized resin was sequentially washed with 10 ml of dichloromethane, methanol, dichloromethane, and dimethylformamide to obtain compound 2a quantitatively.
[0167] Step 2. Compound 2b: Fmoc-Lys(Fmoc)-Hyp(tBu)-O-2-chlorotrityl resin (Fmoc-Lys(Fmoc)-Hyp(tBu)-O-2-chloro trityl resin) synthesis
[0168]
[0169] 5 ml of 20% piperidine (in dimethylforma...
Embodiment 3
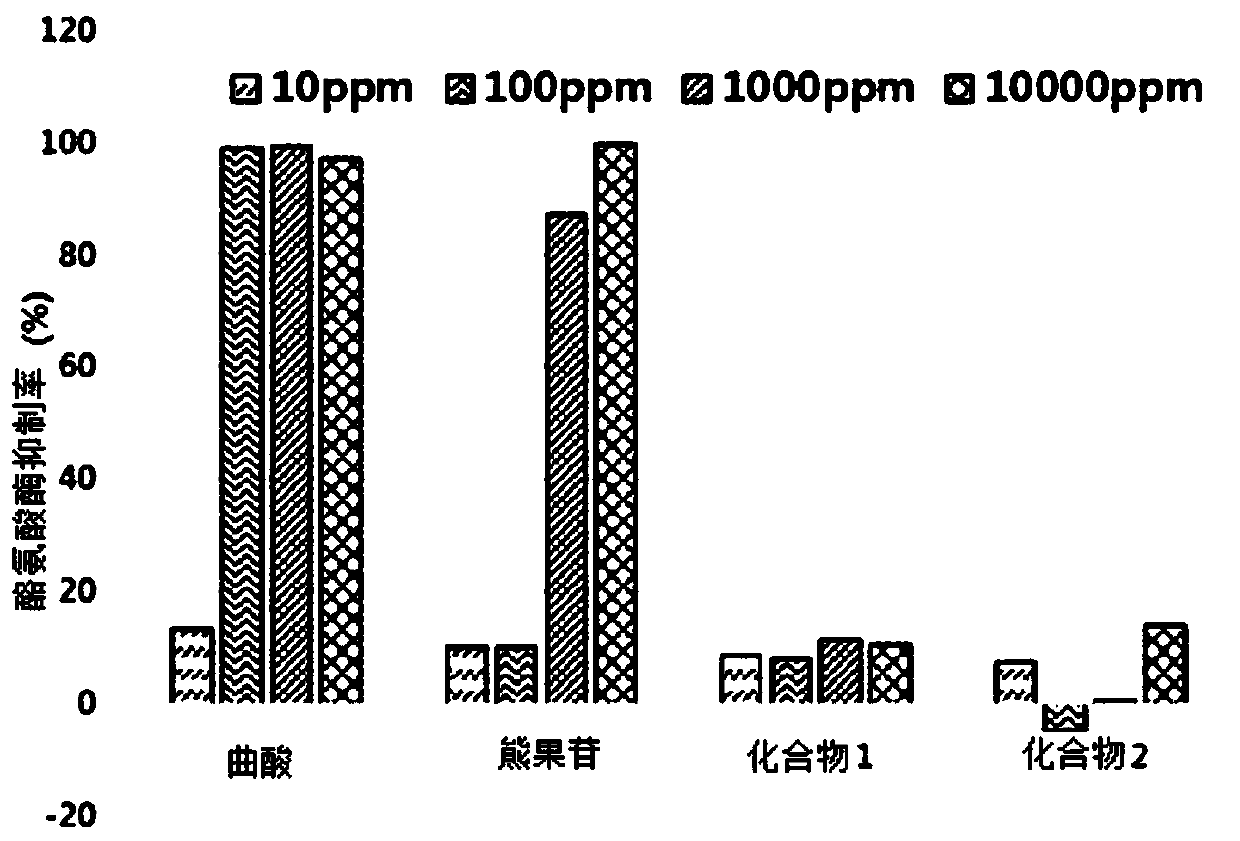
[0176] Example 3. Confirmation of tyrosinase inhibitory ability
[0177] In order to analyze whether Compound 1 and Compound 2 obtained in the above examples inhibit tyrosinase activity, a Tyrosinase inhibition assay (Tyrosinase inhibition assay) was carried out.
[0178] As a specific experimental method, mushroom tyrosinase (Mushroom tyrosinase) was diluted to 2 units / ul in 0.1M sodium phosphate buffer (Sodium phosphate buffer), and tyrosine (Tyrosine) was dissolved in Water becomes 0.03% by weight. Each experimental group described in Table 1 below was also diluted to 10 ppm, 100 ppm, 1000 ppm, and 10000 ppm in 0.1 M sodium phosphate buffer, and mixed as shown in Table 1. React at 37° C. for 1 hour and measure the absorbance (wavelength: 490 nm, Epoch spectrophotometer, BioTek, USA). Based on this result, calculation was performed as the tyrosinase inhibition rate compared to the control group.
[0179] show the result in figure 2 middle.
[0180] as from figure 2 A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com