Lactobacillus casei SYF-08 capable of adsorbing heavy metal lead and application of lactobacillus casei SYF-08
A technology of SYF-08, Lactobacillus casei, applied in Lactobacillus, application, bacteria used in food preparation, etc., can solve the problem of no reported separation, reduce lead absorption and chronic lead poisoning, stabilize content, reduce recognition effects of functional impairment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
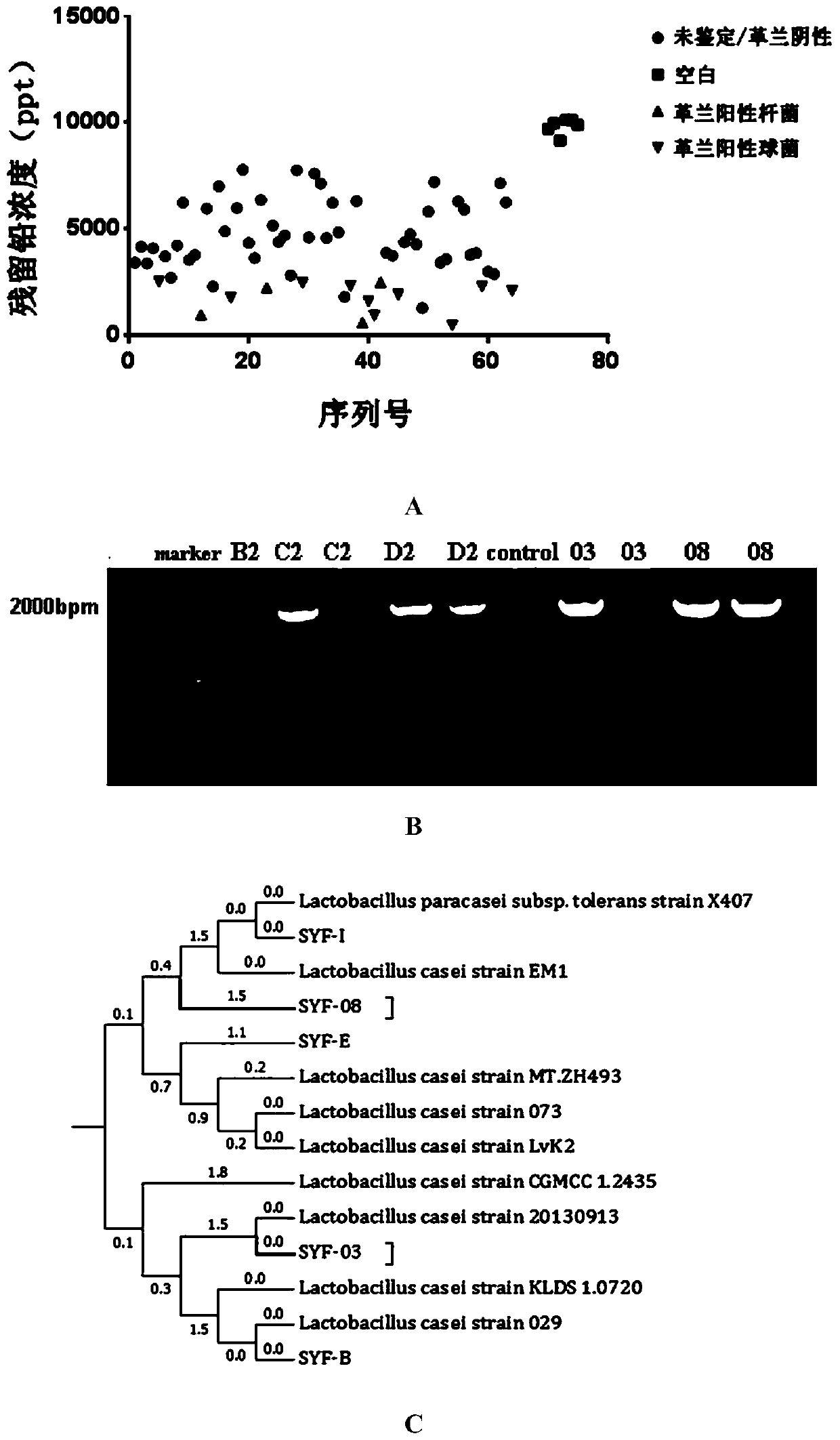
[0029] Isolation of Lactobacillus casei Strains
[0030] Take an appropriate amount of fresh feces of healthy newborns who are exclusively breastfed, and add 5mg / L lead solution to dilute according to the solid-liquid ratio of 1:1000 (g / ml). Take 5 μl sample and inoculate it in the modified MRS medium (central diffusion method) (drawing a plate radially from the center to the edge). 200 μl of lead solution with a concentration of 10,000 mg / L was added dropwise to the center of the plate, and incubated anaerobically at a constant temperature of 37°C for 48 hours. Pick a single colony close to the center of the agar plate and inoculate it into the MRS liquid medium for enrichment culture.
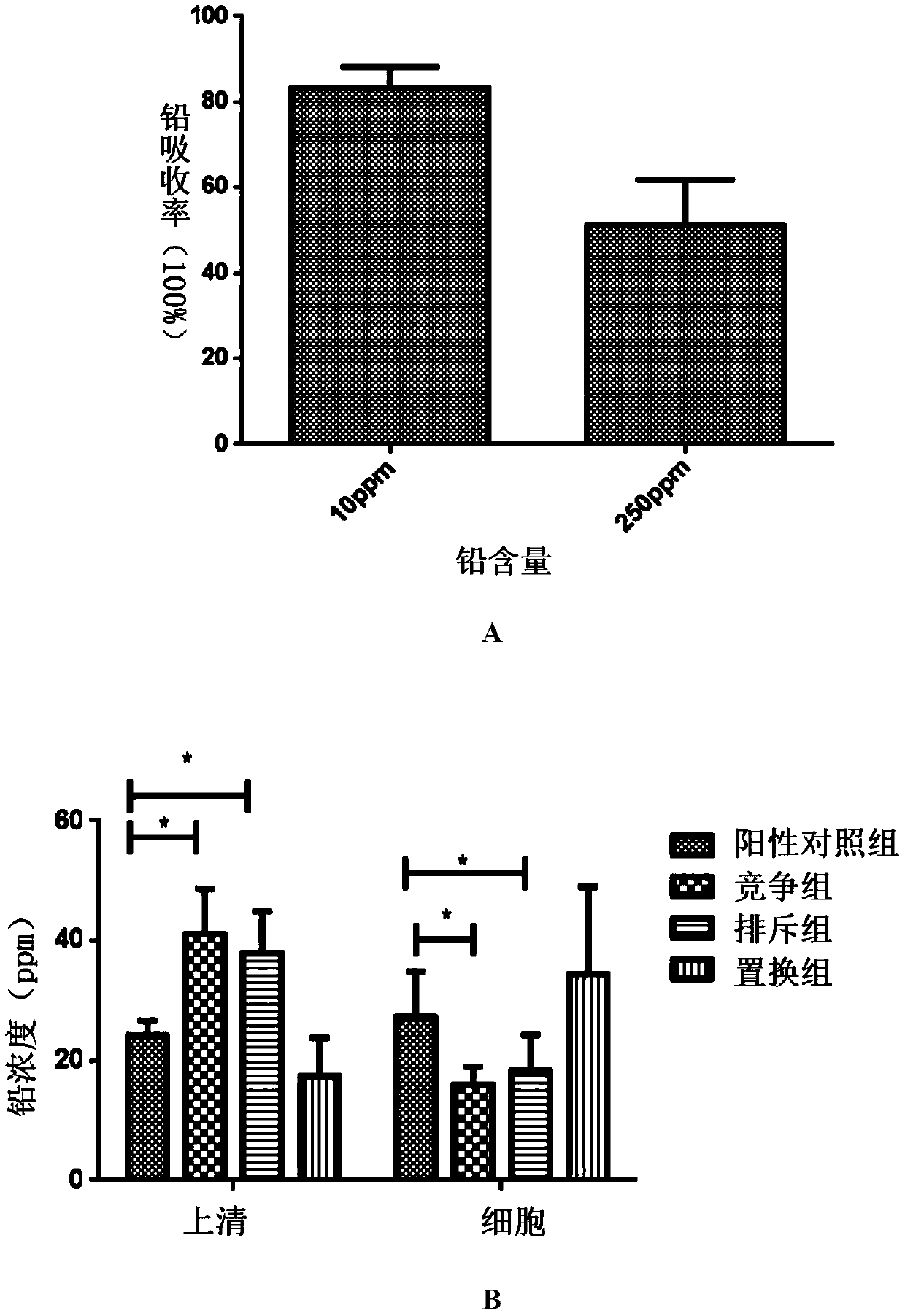
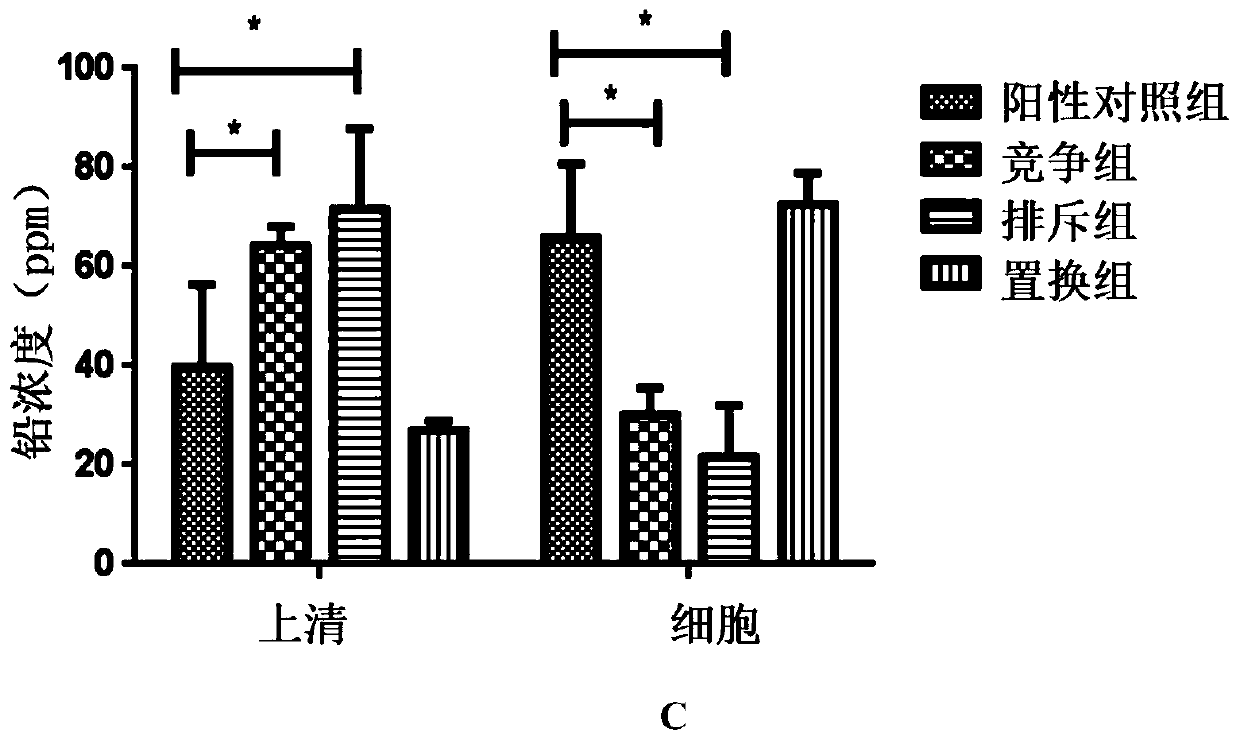
[0031]After culturing for 24 hours, dilute with sterile PBS to prepare a bacterial suspension with a wavelength of 600nm and OD=1; centrifuge (8000rpm, 5min), remove the supernatant, add 1ml of 10ppm lead acetate solution respectively; mix well, and let stand at 37°C for 1h Centrifugal (120...
Embodiment 2
[0065] The test of embodiment 2 Lactobacillus casei SYF-08 probiotic properties
[0066] 1. Acid and bile salt resistance
[0067] Prepare artificial gastric juice with pH value of 2.0, 3.0, 4.0 respectively. Take Lactobacillus casei SYF-08, dilute to 10 with sterile PBS 9 CFU / ml bacterial suspension. Pipette 10 μl of the bacterial suspension into 990 μl of artificial gastric juice with different pH values, incubate anaerobically at 37°C for 1-3 hours, and use the dilution drop plate method to determine the number of viable bacteria at the initial stage and after cultivation.
[0068] Bile resistance test: the bile salt comprehensive test (0.3% bile salt concentration) was carried out on the strains that passed the acid resistance screening. The counted strains were placed in pH 3.0 phosphate buffer, cultured anaerobically at 37°C for 2 hours, inoculated 10 μL in 990 μL solution with a bile salt concentration of 0.3%, cultured anaerobically at 37°C for 8 hours, and determin...
Embodiment 3
[0086] 1. Establishment of chronic lead poisoning mouse model
[0087] 32 6-week-old male mice were fed adaptively for 1 week (lighting cycle 12h, temperature 22-26°C, humidity 40%-60%), were randomly divided into 4 groups, 8 in each group, and processed according to Table 4:
[0088] Table 4 Grouping and treatment of animal experiments
[0089] group Approach PBS group Drinking sterile deionized water and gavage with sterile PBS 100 μl / d for 5 consecutive weeks SYF-08 group Drinking sterile deionized water, SYF-08 10 9 / 100μl / d orally for 5 weeks
Pb group Drinking 100mg / L sterile lead water and gavage with sterile PBS 100μl / d for 5 consecutive weeks SYF-08 group + Pb group Drinking concentration is 100mg / L sterile lead water, SYF-08 10 9 / 100μl / d gavage, continuous gavage for 5 weeks
[0090] 2. Morris water maze test to evaluate the cognitive function of mice
[0091] After gavage, the Morris water maze experiment was carr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com