P-O doped Fe-N-C nanosheet and preparation method thereof
A nanosheet, P-O technology, applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of difficult to achieve excellent dual-functional catalytic performance, large overpotential, poor stability, etc., to achieve catalytic The effect of increasing the number of active sites and increasing the loading of Fe atoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
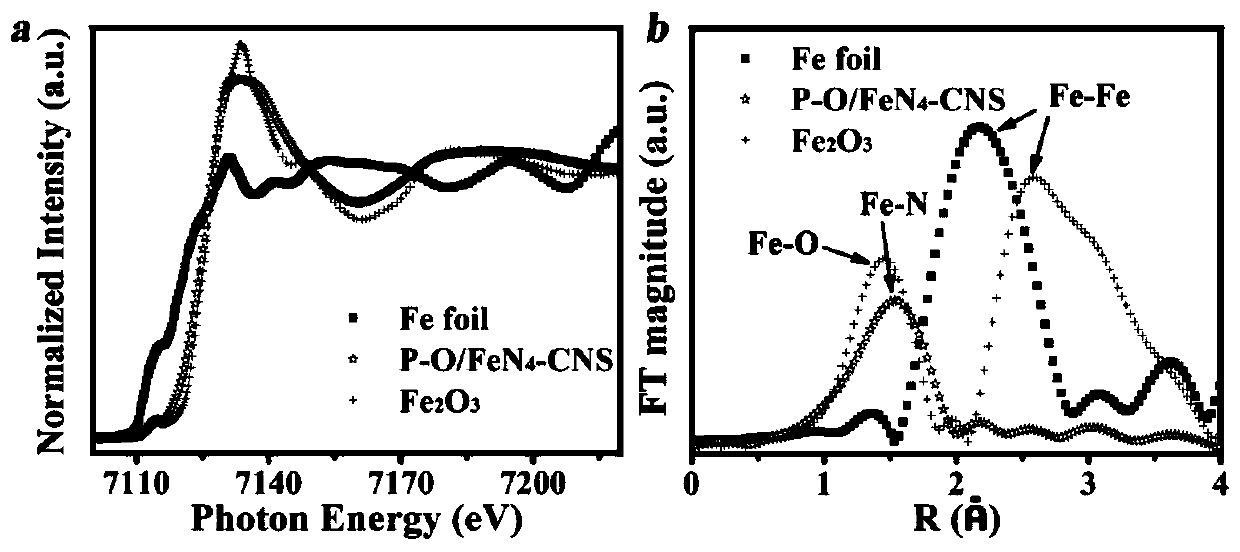
Image
Examples
Embodiment 1
[0035] A preparation method of P-O doped Fe-N-C nanosheets, comprising the following steps:
[0036] Step 1: Add 3g of pyrrole, 0.3g of ferric nitrate and 30g of hydrogen peroxide in sequence in 50ml of deionized water, stir until the color of the solution changes from black to green, dry the solution at 80°C, and prepare powder A;
[0037]Step 2: Add 2.5g of zinc chloride and 0.5g of powder A to 50ml of deionized water in turn, stir until evenly dispersed, place in a freeze dryer, freeze dry to obtain solid B;
[0038] Step 3: Grinding the solid B to powder, carbonizing under the protection of nitrogen atmosphere, the carbonization temperature is 1000°C, the time is 2h, and then cooling down naturally to obtain the solid mixture C;
[0039] Step 4: Add the solid mixture C to deionized water to dissolve, perform suction filtration, and then place it in an oven at 60°C to dry to obtain a black powder D;
[0040] Step 5: Dissolve 0.1g of black powder D and 0.05g of sodium dihyd...
Embodiment 2
[0046] A preparation method of P-O doped Fe-N-C nanosheets, comprising the following steps:
[0047] Step 1: Add 1g of imidazole, 0.1g of ferric chloride and 10g of hydrogen peroxide in sequence in 50ml of deionized water, stir until the color of the solution changes from black to green, dry the solution at 80°C, and prepare powder A;
[0048] Step 2: Add 3g of zinc chloride and 0.5g of powder A in sequence to 50ml of deionized water, stir until evenly dispersed, place in a lyophilizer, and lyophilize to obtain solid B;
[0049] Step 3: Grinding the solid B to powder, carbonizing under the protection of a nitrogen atmosphere, the carbonization temperature is 900°C, and the time is 2 hours, and then cooling down naturally to obtain a solid mixture C;
[0050] Step 4: Add the solid mixture C to deionized water to dissolve, perform suction filtration, and then place it in an oven at 60°C to dry to obtain a black powder D;
[0051] Step 5: Dissolve 0.1g of black powder D and 0.1g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com