Micro-reaction system and method for synthesizing rubber anti-scorching agent CTP
A scorch inhibitor and micro-reaction technology, applied in chemical instruments and methods, chemical/physical/physical-chemical reactors, chemical/physical/physical-chemical stationary reactors, etc., can solve the instability of cyclohexylsulfenyl chloride, Problems such as low overall reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
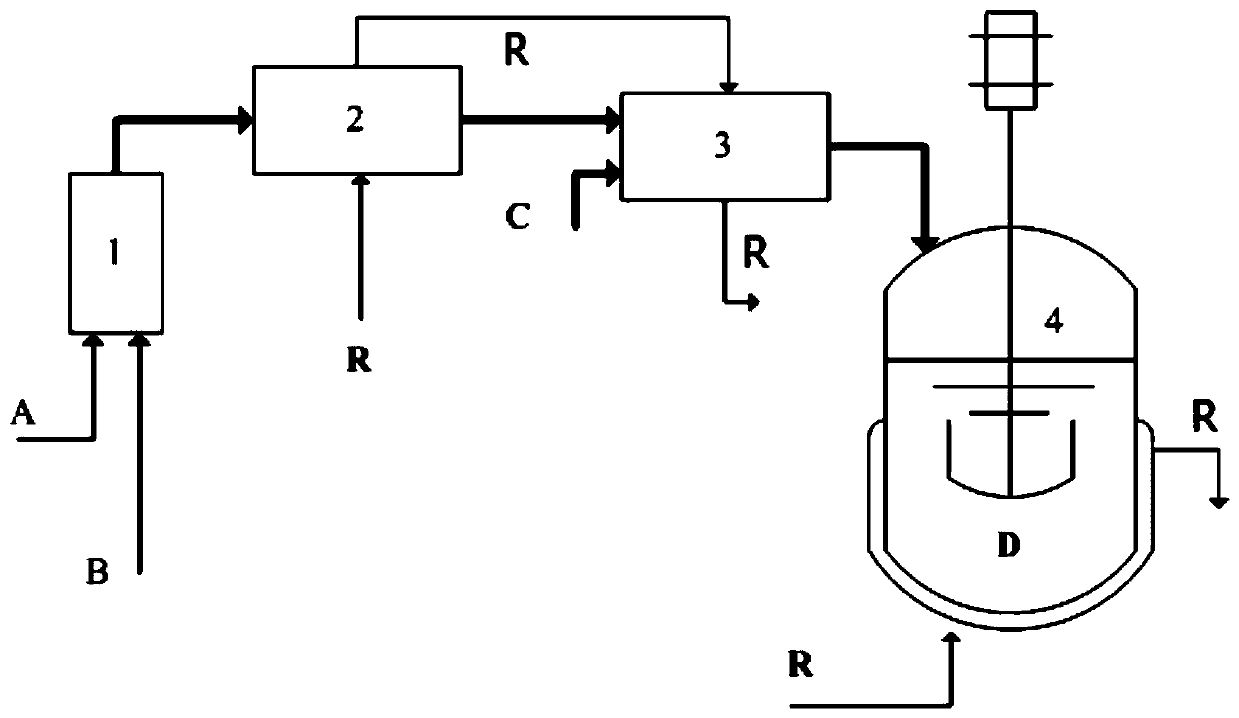
[0032] Microreactor systems such as figure 1Shown, wherein, the effective volume of the internal microchannel of the chlorination microreactor 3 is 2.7 milliliters, the volume of the stirred tank reactor is 1 liter, and the anchor type stirring paddle is equipped. Dicyclohexyl disulfide is delivered by pump 1 with a volume flow rate of 3.4ml / min, and the mixed alkanes of solvent n-heptane and isoheptane are delivered by pump 2 with a volume flow rate of 36.6ml / min. (no heat exchange) mixed to form a dicyclohexyl disulfide solution (mass concentration of the dicyclohexyl disulfide reaction solution can be calculated to be 12%), enter the micro heat exchanger 2 and cool down to minus 10°C, enter the chlorine after cooling The microreactor 3 mixes with the chlorine gas of 330ml / min (standard condition), and the reaction temperature in the control microreactor 3 is subzero 20 ℃, and the reaction time (calculated in liquid disulfide solution) is 4 seconds, and the reaction generate...
Embodiment 2
[0034] Microreactor systems such as figure 1 Shown, wherein, the effective volume of the internal microchannel of the chlorination microreactor 3 is 2.7 milliliters, and the volume of the stirred tank reactor is 1 liter, equipped with stirring paddles. Dicyclohexyl disulfide is delivered by pump 1 with a volume flow rate of 8.6ml / min, and the mixed alkanes of solvent n-heptane and isoheptane are delivered by pump 2 with a volume flow rate of 31.4ml / min. (without heat exchange) mixed to form a dicyclohexyl disulfide solution (mass concentration of dicyclohexyl disulfide reaction solution can be calculated to be 28.5%), enter the micro heat exchanger 2 and cool down to minus 10°C, enter chlorine after cooling The microreactor 3 is mixed with the chlorine gas of 875ml / min (standard condition), and the reaction temperature in the control microreactor 3 is subzero 10 ℃, and the reaction time (in terms of liquid disulfide solution) is 4 seconds, and the reaction generates cyclohexyl...
Embodiment 3
[0036] Microreactor systems such as figure 1 Show, wherein, chlorination microreactor 3 internal microchannel effective volume is 0.3 milliliter (obtained by reducing channel number and number of layers), stirred tank reactor volume 1 liter, is equipped with stirring paddle. Dicyclohexyl disulfide is transported by pump 1 with a volume flow rate of 8.6ml / min. The mixed alkanes of n-heptane and n-octane as solvent are transported by pump 2 with a volume flow rate of 31.4ml / min. (without heat exchange) mixed to form a dicyclohexyl disulfide solution (mass concentration of dicyclohexyl disulfide reaction solution can be calculated to be 28.5%), enter the micro heat exchanger 2 and cool down to minus 5°C, enter chlorine after cooling The microreactor 3 is mixed with the chlorine gas of 875ml / min (standard condition), and the reaction temperature in the control microreactor 3 is 0 ℃, and the reaction time (in terms of liquid disulfide solution) is 0.45 seconds, and the reaction gen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com