Application of Akkermansia Muciniphila in preparing anti-depression drug or health-care product
A technology of health care products and medicines, applied in the field of probiotics, to achieve good control effects, safe use, and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. The preparation method of Akkermansia Muciniphila bacterial liquid:
[0033] Take 1ml of Akkermansia Muciniphila bacteria solution, add it into 10ml of brain heart extract broth, incubate at 37°C for 72 hours under anaerobic environment, centrifuge the bacteria solution (6000rpm) for 20 minutes, separate the supernatant, and dissolve it with sterile PBS solution. The bacterial solution was adjusted to OD600=1 to obtain the Akkermansia Muciniphila bacterial solution (abbreviated as AKK bacterial solution).
[0034] 2. Preparation method of pasteurized AKK bacteria (dead bacteria)
[0035] Place the AKK bacterial solution obtained in step 1 in a water bath and heat to 62-65°C for 30 minutes.
[0036] 3. Preparation method of Amuc_1100
[0037] By amplifying the gene encoding the intracellular part of 1100, the resulting PCR product was cloned into the pET32aTEV vector by restriction enzyme ligation to construct the expression plasmid of Amuc_1100 fused to the His tag...
Embodiment 2
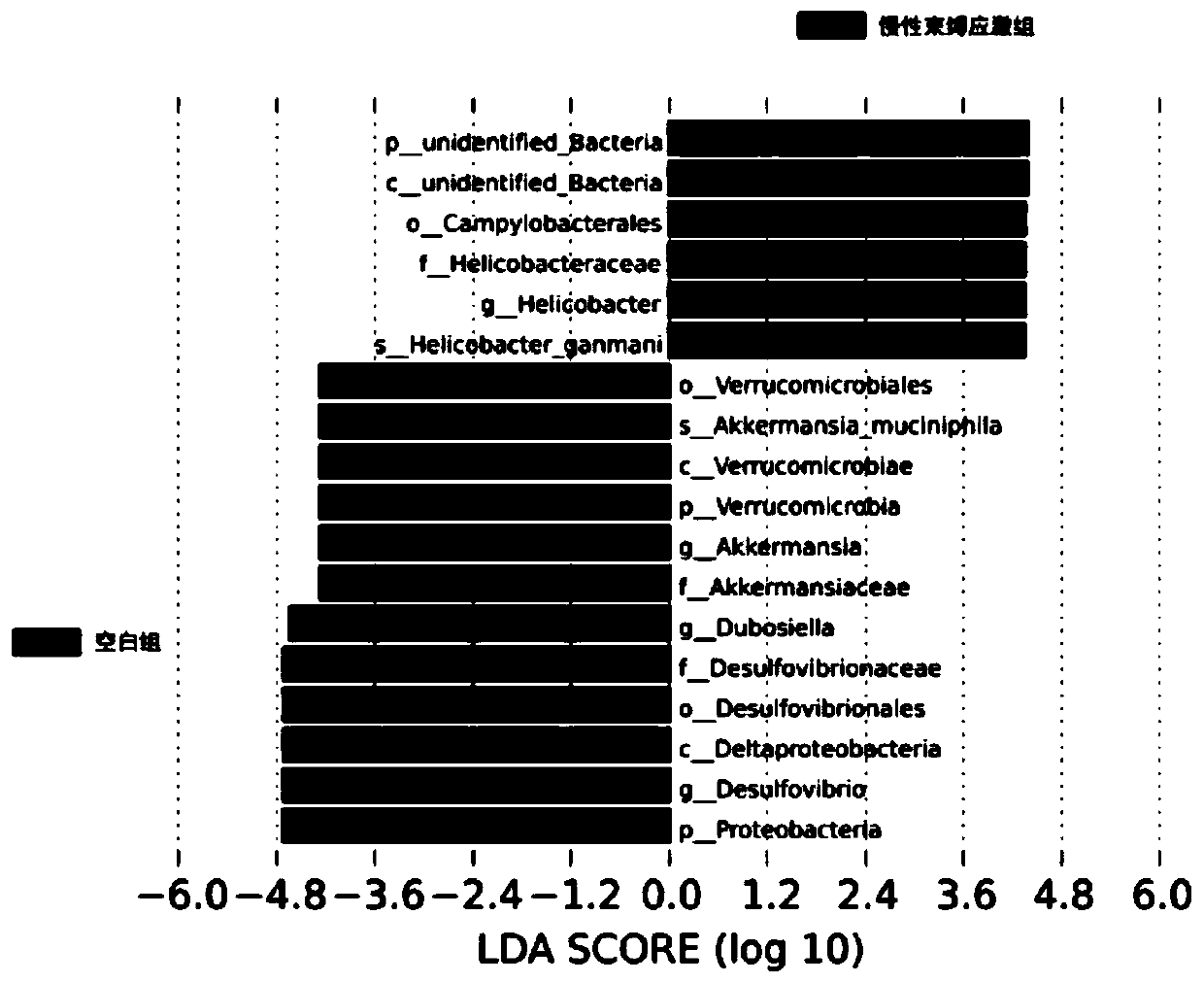
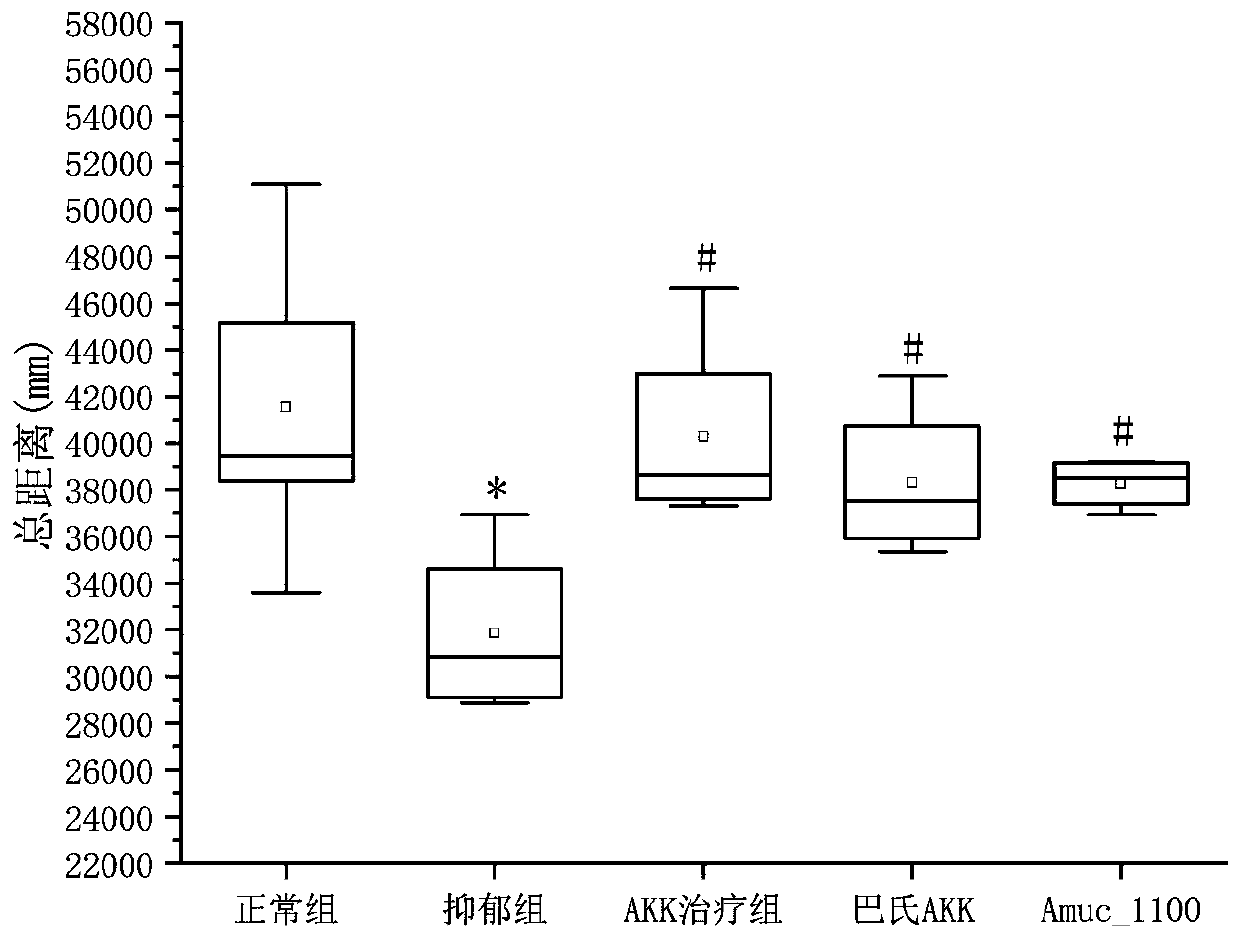
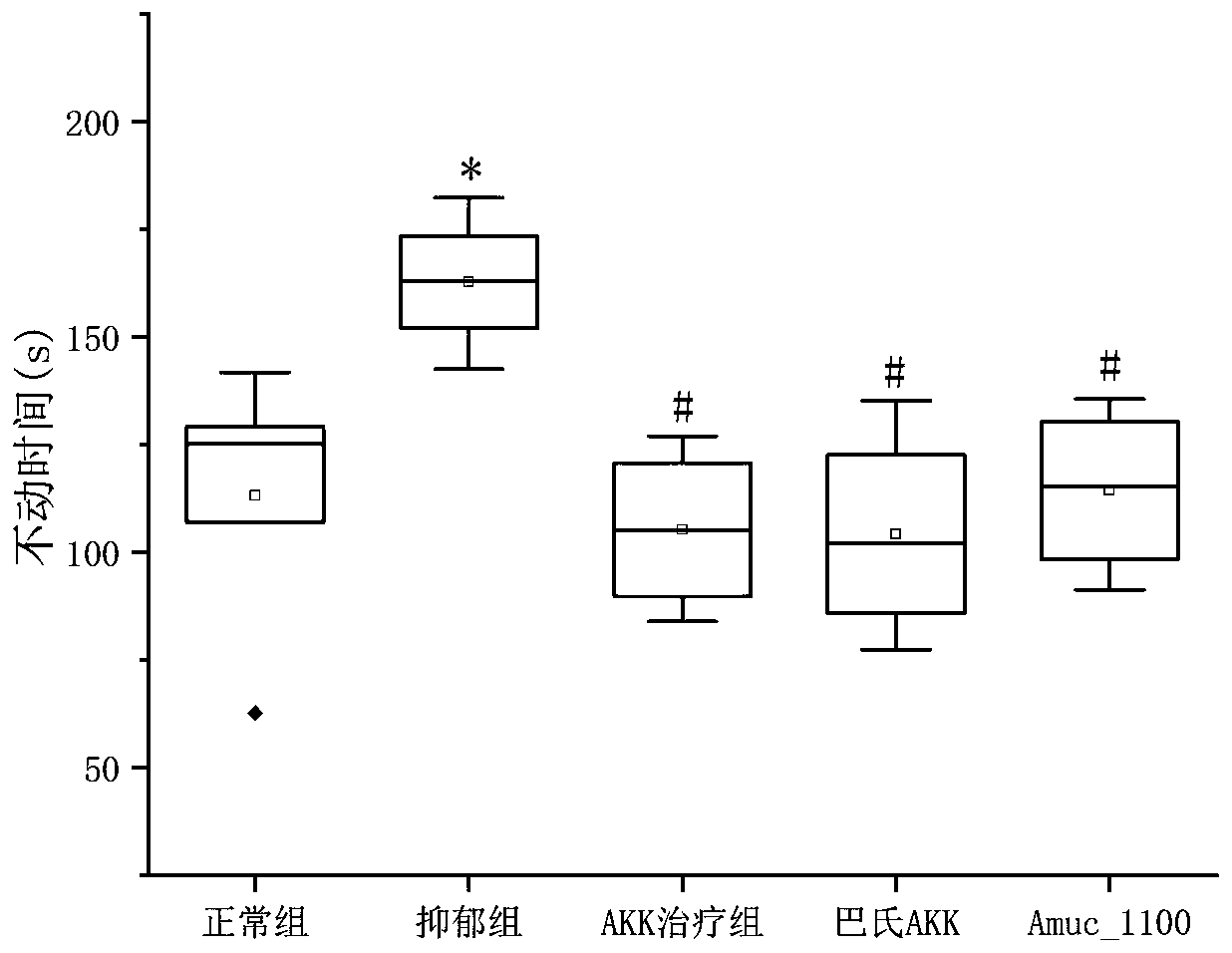
[0074] Example 2 investigates the therapeutic effect of AKK bacteria on depression-complicated enteritis model mice
[0075] 1. Construction of chronic restraint stress depression mouse model (Chronic restraint stress, CRS) The specific modeling method of CRS is as follows: take male C57BL / 6 mice, aged 6-8 weeks. After one week of adaptive feeding, the mice were placed in a 0.5mm diameter, 50ML centrifuge tube for 3 hours every day for 30 days. Behavioral test results were recorded on day 30. During the CRS modeling period, the mice in the other groups were restricted from feeding and watering; at the end of the experiment, behavioral tests were performed on all mice, including: open field test, tail suspension test and forced swimming test. After the behavioral test showed that the depression model was successfully established, the mice were sacrificed immediately and the contents of the cecum were removed. The caecal contents of 3 mice were randomly mixed, dissolved in ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com