Cholesterol silicon phthalocyanine (Chol-SiPc) nano-systems capable of serving as living-cell cholesterol zone and Golgi body probe, preparation method for Chol-SiPc nano-systems and application of Chol-SiPc nano-systems
A Golgi and cholesterol technology, applied in the fields of nanotechnology, chemical instruments and methods, and nanotechnology for materials and surface science, can solve the problems of high price, easy bleaching, incomplete clarity of Filipin staining cholesterol, etc. Aggregation behavior, improvement of stability, and effect of improving the efficiency of reactive oxygen species generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0024] 1) Dichlorosilicon (IV) phthalocyanine (SiPcCl 2 )Synthesis
[0025] Add 1,3-diiminoisoindoline (7.28 g, 50.15 mmol), silicon tetrachloride (8.3 mL) and quinoline (83 mL) respectively into a three-necked flask, stir and reflux for 30 min at 220 °C , cooled to room temperature, poured into 500 mL of methanol solution, stirred and allowed to stand for about 1 hour, filtered, and the filter residue was washed with 35 mL each of acetone, methanol, dichloromethane, methanol and other solvents, and dried to obtain a purple-red solid (2 Chlorosilicon (IV) phthalocyanine) 3.6759 g, yield 48.62%.
[0026] ) Synthesis of cholesteryl silicon phthalocyanine (Chol-SiPc)
[0027] Cholesterol (CAS: 57-88-5) (0.115 g, 0.3 mmol), dichlorosilicon(IV) phthalocyanine (0.0611 g, 0.1 mmol) from step 1) Anhydrous potassium carbonate (0.276 g, 2 mmol) Add 20 mL of toluene to a 100 mL reaction flask, and reflux for 24 hours at 120 °C. Cooled to room temperature, filtered, and the filtrate w...
specific Embodiment 2
[0034] Concrete steps are the same as embodiment one: K in process 2) 2 CO 3 (0.09 g, 0.654 mmol) was changed to NaH (0.01 g, 0.654 mmol), and other reaction conditions were the same. 6.6 mg of blue-purple solid (cholesteryl silicon phthalocyanine (Chol-SiPc)) was obtained with a yield of 2.4%.
[0035] Process 2) K 2 CO 3 (0.09 g, 0.654 mmol) was changed to pyridine (0.05 g, 0.654 mmol), and other reaction conditions were the same. 5.8 mg of blue-purple solid (cholesteryl silicon phthalocyanine (Chol-SiPc)) was obtained with a yield of 1.46%.
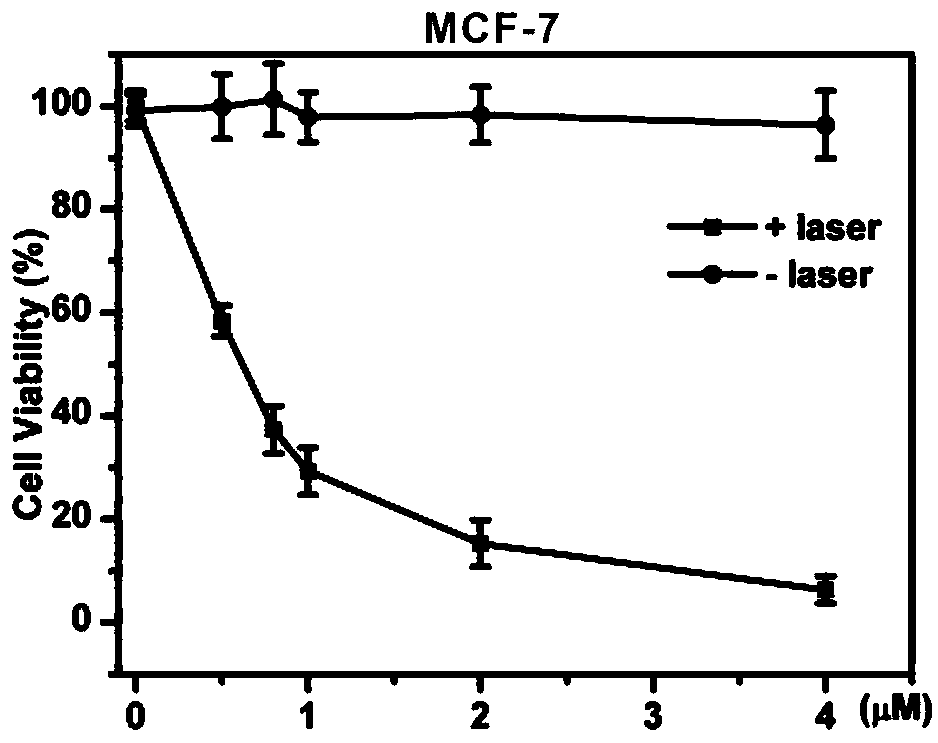
[0036] ) Real-time tracking of breast cancer cells MCF-7 and MDA-MB-231 on FITC-DSPE@Chol-SiPc
[0037] MCF-7 and MDA-MB-231 cells in the logarithmic growth phase were taken, digested, collected by centrifugation, resuspended and counted, and approximately 1×10 4 The concentration was placed in a 3.5 cm confocal dish, placed at 37°C, 5% CO 2 Cultivate overnight (20-24 h) in a constant temperature incubator. The next day, the cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com