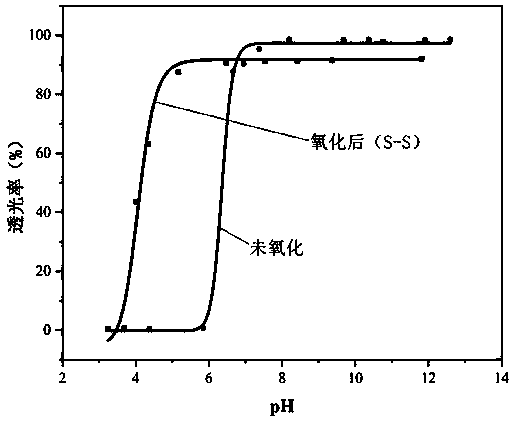
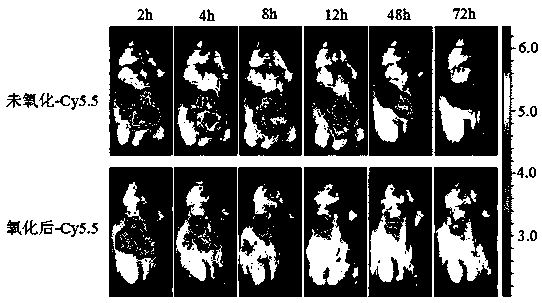
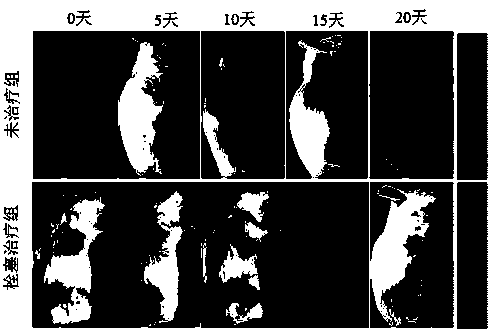
PH-reduction dual-response polymer embolization agent for tumor catheter-free embolization and synthesis thereof
An embolic agent and double-response technology, which is applied in the field of polymer embolic agent and its synthesis for pH-reduction dual-response tumor catheter-free embolization, can solve problems such as gelation in the tube, simplify the operation process and requirements, and increase compliance , the effect of reducing height requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add 1g of newly prepared L-threonine NCA to a 50mL three-necked flask, dissolve in 3mL redistilled 1,4-dioxane, and 2 Add 2.835 mg of piperazine under protective conditions to carry out the polymerization reaction. After the polymerization reaction is completed, add ethanol to obtain a white precipitate, centrifuge, and wash the white precipitate with ethanol and ether for 2 to 3 times, and dry it in vacuum at 40°C for 24 hours. White poly (L-threonine) (PLGThr) was obtained. Add 0.4 g of PLGThr to a 50 mL three-necked flask, suspend it in 2 mL of 1,4-dioxane, and 2 Added newly prepared L-glutamic acid-5-benzyl ester-NCA (BLG-NCA) and L-cysteine NCA (LCys-NCA) under protective conditions, reacted for 72 hours, and precipitated with absolute ethanol to obtain a white solid as poly (L-glutamic acid-5-benzyl ester-L-cysteine)-poly(L-threonine)-poly(L-glutamic acid-5-benzyl ester-L-cysteine)( P(BLG-LCys)-PLGThr-P(BLG-LCys)), filtered, and then added to a round-bottomed ...
Embodiment 2
[0060] Add 0.8g of fresh L-threonine NCA to a 50mL three-necked flask, dissolve it with 3mL redistilled 1,4-dioxane, and 2 Add 0.42 mL of 1,4-cyclohexanediamine in 1,4-dioxane solution with a mass volume concentration of 5.4 mg / mL under protective conditions, react at room temperature for 48 hours, add ethanol to obtain a white precipitate, centrifuge, wash with ethanol, ether Wash 2-3 times, and dry in vacuum at 40°C for 24 hours to obtain white poly(L-threonine) (PLGThr); add 0.4 g of PLGThr into a 50 mL three-necked flask and suspend it in 2 mL of 1 ,4-dioxane, in N 2 Add 0.35g of fresh L-glutamic acid-5-benzyl ester-NCA (BLG-NCA) and 0.28g of L-tyrosine NCA (LTyr-NCA) under protective conditions, react at 35°C for 48 h, and use anhydrous Ethanol precipitation, the solid white obtained is poly(L-glutamic acid-5-benzyl ester-L-tyrosine)-poly(L-threonine)-poly(L-glutamic acid-5-benzyl ester- L-Tyrosine) (P(BLG-LTyr)-PLGThr-P(BLG-LTyr)), filtered, and then added to a round-b...
Embodiment 3
[0065] Add 2mL of triethylamine and 1,4-dioxane mixed solution in 50mL three-necked flask, the volume ratio of triethylamine and 1,4-dioxane in this mixed solution is 25: 1, in N 2 Add 0.35g of newly prepared L-glutamic acid-5-benzyl ester-NCA (BLG-NCA) and 0.20g of L-cysteine NCA (LCys-NCA) under protective conditions, react at 35°C for 48 h, and use Precipitate with water and ethanol, the obtained white solid is poly(L-glutamic acid-5-benzyl ester-L-cysteine) [P(BLG-LCys)], wash with ethanol and ether three times respectively, and CIA at 40°C Vacuum dried for 24h. Add 0.5g of P(BLG-LCys) into a 50 mL three-neck flask filled with 5 mL of DMF, dissolve it, and 2 Add 0.25g polyethylene glycol 1000 and 2mL hydrogen bromide aqueous solution under protective conditions, and reflux at 80°C for 8h; add hydrochloric acid aqueous solution with a pH value of 2 to obtain a large amount of white precipitate, filter, and then add 30 mL of the mixed solution to the round bottom In the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com