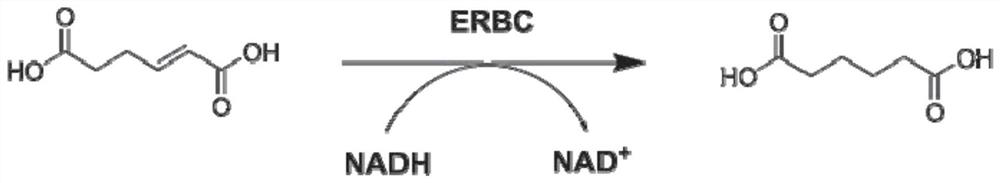
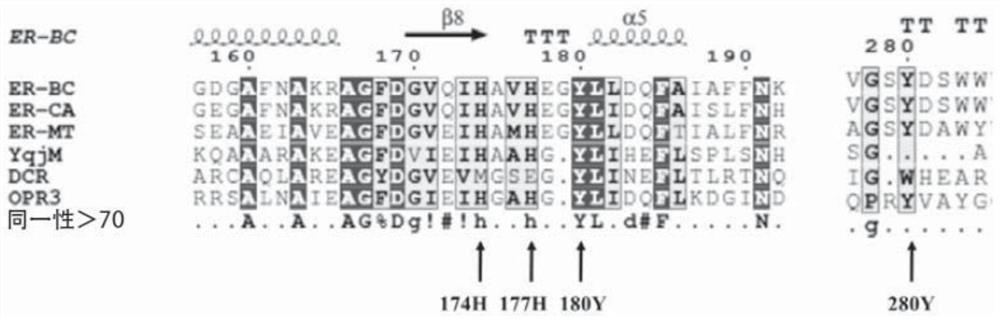
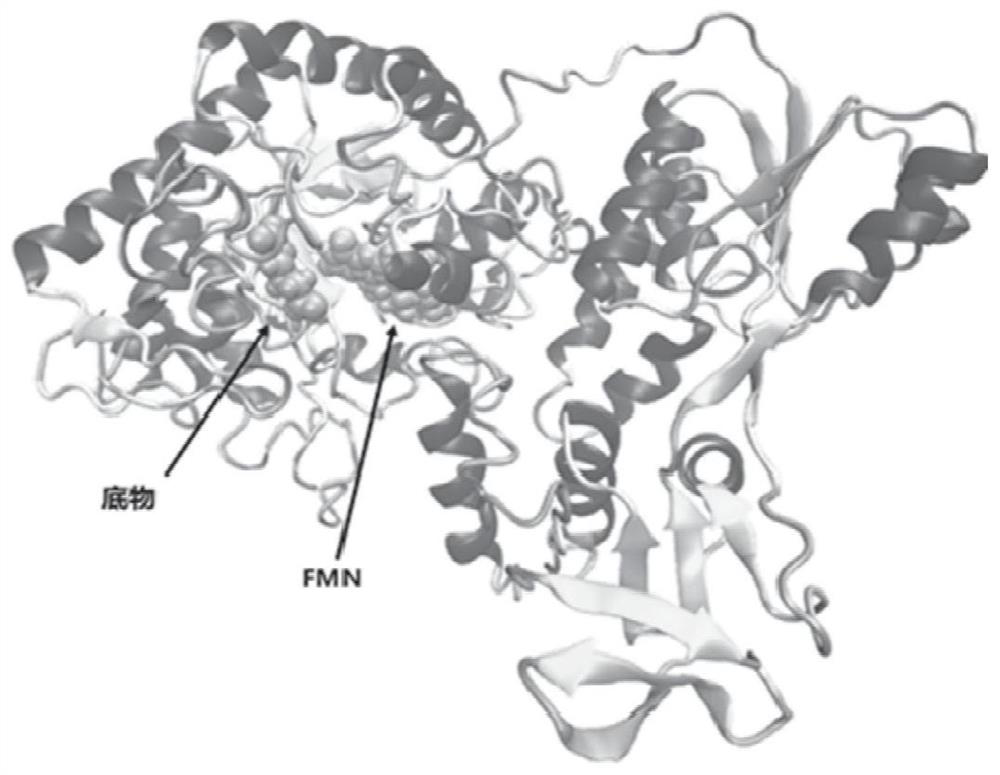
A mutant protein of enoate reductase and its application
A technology of mutants and reductases, which is applied in the field of directed evolution transformation of enzymes and biocatalysis applications, and can solve problems such as low catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0060] The existing enoate reductase ER-BC has a low catalytic efficiency to 2-ene adipate. In order to solve this problem, the inventors have conducted a lot of research on the enoate reductase ER-BC, and through semi-rational design evolution, A mutant protein of enoate reductase ER-BC with higher activity was obtained.
[0061] Furthermore, based on the enoate reductase gene (GenBank accession number CP003056.1) of Bacillus coagulans 36D1, the present inventors used error-prone PCR and semi-rational design methods, through random mutation and site-directed mutation, to Evolution has resulted in a mutant protein of the enoate reductase ER-BC.
[0062] Specifically, the inventors obtained a mutant protein of enoate reductase ER-BC with higher activity through the following steps:
[0063] (1) Based on the enoate reductase gene (GenBank accession number CP003056.1) of Bacillus coagulans 36D1, a random mutation library was constructed by error-prone PCR, and mutants with impro...
Embodiment 1
[0113] Example 1: Construction of error-prone PCR mutation library
[0114] Using the error-prone PCR kit (GeneMorph II Random Mutagenesis Kit (Agilent Technologies)), construct an Error prone with a base mutation rate of 1-2‰ (2-4 bases / gene) and an amino acid mutation rate of 1-2 amino acids / gene PCR mutation library.
[0115] The error-prone PCR reaction system is shown in Table 1, with a total volume of 50 μL.
[0116] Table 1 Error-prone PCR reaction system
[0117]
[0118] Wherein, the F and R primer sequences are:
[0119] Upstream primer F: 5′-CGCGGATCCATGAAGTACAAGAAGCTGTTCG-3′
[0120] Downstream primer R: 5′-CCCAAGCTTTTACAGGTTTGCAGCGACC-3′
[0121] The amplification program of error-prone PCR is shown in Table 2:
[0122] Table 2 Amplification program of error-prone PCR
[0123]
[0124]
[0125] After the error-prone PCR, the PCR products were separated and checked by agarose gel electrophoresis, and the gene DNA fragments required for the experiment...
Embodiment 2
[0126] Example 2: Screening of High Enzyme Activity Mutants in Error-prone PCR Mutation Library
[0127] It is known from the literature that NADH is required for ER-BC catalysis (Ress T, HummelW, Hanlon S.P, etal. ChemCatChem 2015, 7, 1302-1311), so it can be determined by UV / Vis-based method (UV / Vis absorption spectroscopy) The reduced amount of NADH is used for high-throughput screening of high-enzyme activity mutants.
[0128] 96-well plate primary screening:
[0129] (1) Prepare a sterilized 96 deep-well plate and HB-PET self-induction medium in advance, and fill the 96-deep-well plate with HB-PET self-induction medium with 0.1% Kana antibiotics in the ultra-clean bench, 700 μL per well;
[0130] (2) Label the single clones on the plate, then pick them into 96 deep-well plates one by one, and culture them at 30°C / 180rpm for 24h. Each deep-well plate has 6 unmutated single clones as a control;
[0131] (3) After the cultivation, put the 96 deep-well plate containing the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com