Stable triazinone anticoccidial drug dry suspension and preparation method thereof
A technology of triazine ketones and dry suspensions, which can be applied in directions such as pharmaceutical combinations, pharmaceutical formulations, active ingredients of heterocyclic compounds, etc., can solve the problems of complex preparation process and high equipment conditions, and achieve a simple preparation process and high safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
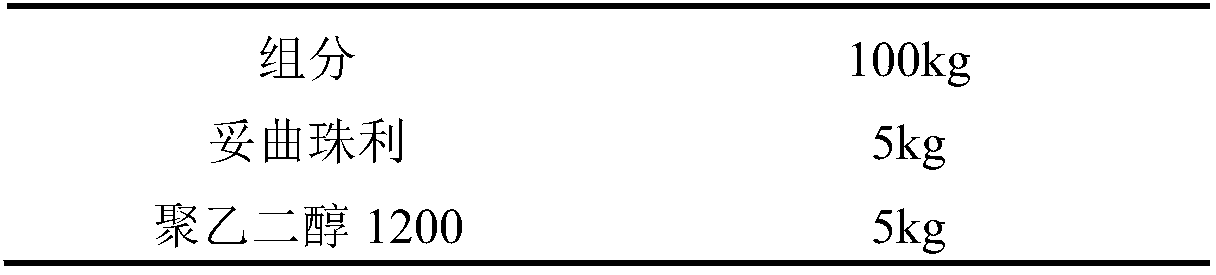
[0022] Embodiment 1: Triazinone anticoccidial drug dry suspension formulation:
[0023]
[0024]
[0025] Preparation method:
[0026] (1) Weigh Polyethylene Glycol 1200, heat to 60°C, reach a stirrable state, then add 5 kg of Toltrazuril, stir to make it evenly dispersed, and it is Suspension A;
[0027] (2) Put suspension A under high shear at 60°C for 5 minutes, then add it into microcrystalline cellulose, and stir to make a soft material;
[0028] (3) The above-mentioned soft material is granulated by an extrusion spheronizer, and the obtained granules are dried, crushed and passed through a 60-mesh sieve, and the content is measured, and the prescribed amount of anhydrous glucose and lactose is added, mixed evenly, and packaged, that is have to.
Embodiment 2
[0029] Embodiment 2: prescription of triazinone anticoccidial drug dry suspension:
[0030]
[0031] Preparation method:
[0032] (1) Weigh 1 kg of sodium lauryl sulfate, add it to 16 L of 20% ethanol aqueous solution, stir evenly, add Patopazuril 6 kg, stir to make it evenly dispersed, and it is suspension A;
[0033] (2) Suspension A was subjected to high shear for 15 minutes;
[0034] (3) Add the above solution into the maltodextrin and stir to make a soft material;
[0035] (4) The above-mentioned soft material is granulated by an extrusion spheronizer, and the obtained granules are dried, pulverized and passed through a 60-mesh sieve, and the content is measured, added to anhydrous sodium sulfate in the prescribed amount, mixed evenly, and packaged to obtain final product .
Embodiment 3
[0036] Embodiment 3: prescription of triazinone anticoccidial drug dry suspension:
[0037]
[0038] Preparation method:
[0039] (1) Take 20kg Polyethylene Glycol 600, add Patopazuril, stir to obtain mixture A;
[0040] (2) After high-shearing the above solution for 2 minutes, add microcrystalline cellulose and stir to make a soft material;
[0041] (4) The above-mentioned soft material is granulated by an extrusion spheronizer, the obtained granules are dried, crushed and passed through a 60-mesh sieve, the content is measured, added to the prescribed amount of lactose, mixed evenly, and packaged to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com