Synthetic method of beta, beta-disubstituted propionate derivative
A synthesis method and technology of propionate, applied in the beta field, can solve the problems of narrow substrate range, poor functional group tolerance, inability to obtain diverse structures, etc., to achieve the effects of easy reaction, reduction of synthesis steps, and rich applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
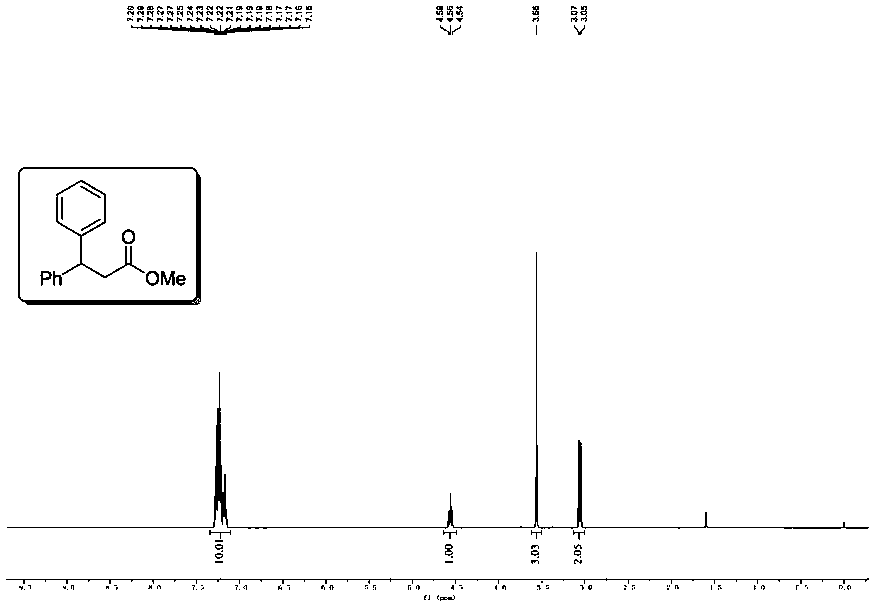
[0044] Example 1 At 25°C, in a glove box under an argon atmosphere, three (dibenzylidene indeneacetone) dipalladium Pd 2 (dba) 3 (4.6mg, 0.005mmol), 1,3-bis(2,6-diisopropylphenyl) imidazolium chloride (6.3mg, 0.015mmol), α,α,α-terpyridine (2.3mg, 0.01 mmol), THF (2 mL) was added to an oven-dried microwave tube (10 mL). Next iodobenzene (0.2 mmol) was added and the solution turned from brown to green. Subsequently, MeOH (100 uL), cinnamaldehyde (0.1 mmol), KOtBu (16.8 mg, 0.15 mmol) were gradually added to the mixture (after adding KOtBu, the reaction solution changed from green to gray). Seal the microwave tube with an aluminum cap fitted with a rubber septum and remove it from the glove box. Stir at room temperature 25°C for 12 hours. The cap was removed, the reaction mixture was exposed to the air, and filtered through a silica gel column (rinsed with 5 mL of ethyl acetate) to obtain a dark brown solution. Volatile materials were removed using a rotary evaporator. The ...
Embodiment 2
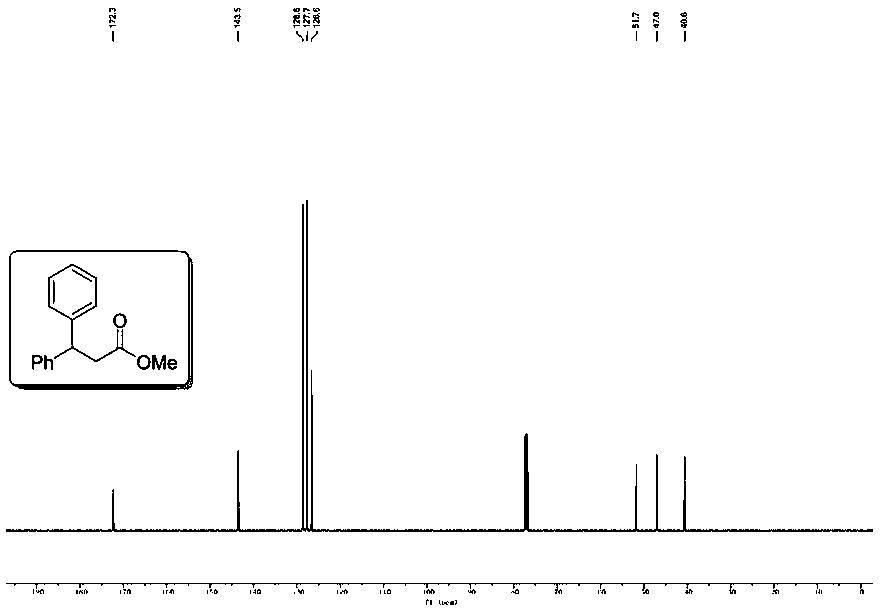
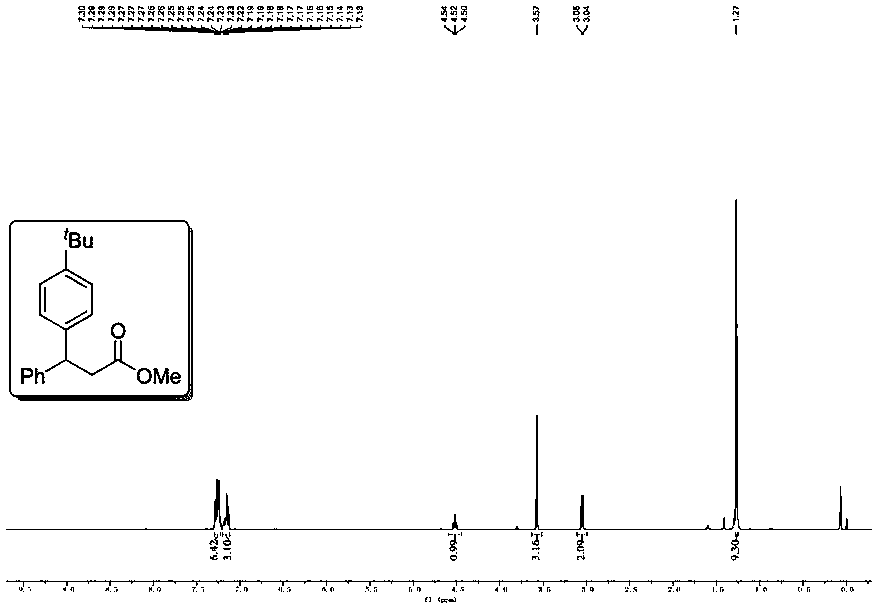
[0051] Example 2 Use 4-tert-butyl iodobenzene (52.0mg, 0.2mmol) to replace the iodobenzene in Example 1, the remaining operations are the same as in Example 1, and the crude product is purified by flash column chromatography (petroleum ether: ethyl acetate = 100:1), to obtain the corresponding colorless oil product 2 (20.1mg, 68% yield), the proton spectrum and carbon spectrum nuclear magnetic resonance spectrum of the product are respectively as follows Figure 2A and Figure 2B As shown, the spectrogram data is: 1 H NMR (400MHz, CDCl 3 )δ7.30–7.22(m,6H),7.20–7.12(m,3H),4.52(t,J=8.0Hz,1H),3.57(s,3H),3.05(d,J=8.0Hz,2H ),1.27(s,9H)ppm; 13 C{ 1 H}NMR (101MHz, CDCl 3 )δ172.5, 149.3, 143.7, 140.5, 128.6, 127.8, 127.3, 126.6, 125.6, 51.8, 46.6, 40.7, 34.5, 31.4ppm.
Embodiment 3
[0052] Example 3 Use 4-methoxy iodobenzene (46.8mg, 0.2mmol) to replace the iodobenzene in Example 1, the rest of the operations are the same as in Example 1, and the crude product is purified by flash column chromatography (petroleum ether: ethyl acetate = 70:1), to obtain the corresponding colorless oil product 3 (17.5mg, 65% yield), the proton spectrum and carbon spectrum nuclear magnetic resonance spectrum of the product are respectively as follows Figure 3A and Figure 3B As shown, the spectrogram data is: 1 H NMR (400MHz, CDCl 3 )δ7.30–7.25(m,2H),7.23–7.12(m,5H),6.87–6.73(m,2H),4.50(t,J=8.0Hz,1H),3.75(s,3H),3.57 (s,3H),3.02(d,J=8.0Hz,2H)ppm; 13 C{ 1 H}NMR (101MHz, CDCl 3 )δ171.3, 157.1, 142.8, 134.6, 127.6, 127.5, 126.5, 125.4, 112.9, 54.2, 50.6, 45.1, 39.7ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com