Method for separating 4-cyanopyridine by cooling solvent crystallization
A cyanopyridine and cooling crystallization technology, which is applied in the field of separation and purification of chemical production, can solve the problems of unsatisfactory separation effect of 4-cyanopyridine, fumaric nitrile prone to polymerization reaction, and clogging of rectification device equipment, etc. Good economy, reasonable structure, and the effect of improving productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
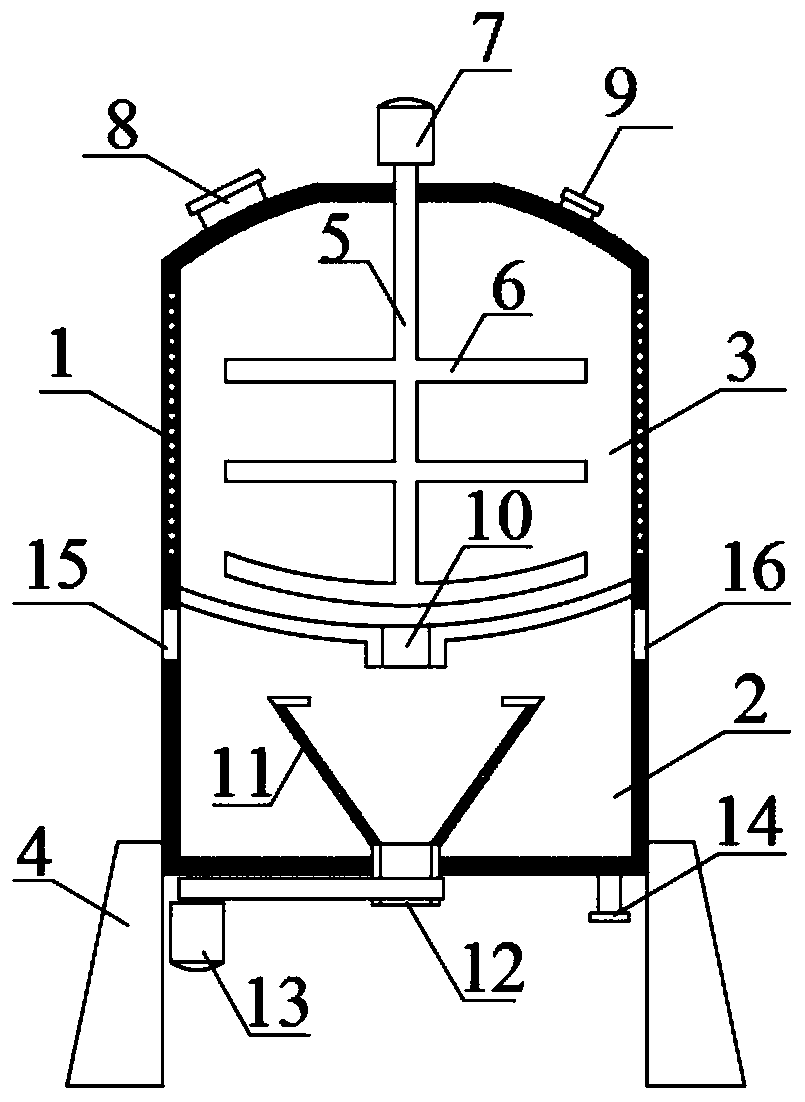
[0024] Such as figure 1 As shown, a 4-cyanopyridine separation and purification device includes a device body 1, a crystallization box 2 and a centrifuge box 3 are arranged inside the device body 1, the crystallization box 2 is fixedly installed above the centrifuge box 3, and the bottom of the centrifuge box 3 is fixed and installed 4. There is a rotating shaft 5 inside the crystallization box 2. The lower end of the rotating shaft 5 is fixed with a stirring rod 6. The upper end of the rotating shaft 5 runs through the middle of the top of the crystallization box 2. The rotating shaft 5 is connected with the crystallization box 2 in a sealed rotation, and the middle of the top of the crystallization box 2 is fixed. Motor one 7 is installed, and the output shaft of rotating shaft 5 tops is connected with motor one 7, and crystallization box 2 top left side is provided with feed inlet 8, and crystallization box 2 top right sides is provided with water inlet 9, and crystallizatio...
Embodiment 2
[0026] A method of using the solvent cooling crystallization of separation and purification device in embodiment 1 to separate 4-cyanopyridine, comprising the following steps:
[0027] S1. Add the crude 4-cyanopyridine to the separation and purification device, add a mixed solvent of ethanol and ether with a volume ratio of 2.5:1 to the separation and purification device, and the mass ratio of the added mixed solvent to the crude 4-cyanopyridine is 1.5: 1. Heat and stir to make a saturated solution of 4-cyanopyridine;
[0028] S2. Cool the saturated 4-cyanopyridine solution prepared in step S1 at a rate of 1.4° C. / min, and 4-cyanopyridine crystals are precipitated to obtain a mixed slurry;
[0029] S3. Centrifugally filter the mixed slurry obtained in step S2 to obtain filter residue 1 and filtrate 1. Clean filter residue 1 with pure water for 1-2 times and then centrifugally filter to obtain filter residue 2 and filtrate 2. Dry filter residue 2 to obtain high-purity 4-cyanop...
Embodiment 3
[0034] A method of using the solvent cooling crystallization of separation and purification device in embodiment 1 to separate 4-cyanopyridine, comprising the following steps:
[0035] S1. Add the crude 4-cyanopyridine to the separation and purification device, add a mixed solvent of ethanol and ether with a volume ratio of 3:1 to the separation and purification device, and the mass ratio of the added mixed solvent to the crude 4-cyanopyridine is 1.5: 1. Heat and stir to make a saturated solution of 4-cyanopyridine;
[0036] S2. Cool the saturated 4-cyanopyridine solution prepared in step S1 at a rate of 1 °C / min, and 4-cyanopyridine crystals are precipitated to obtain a mixed slurry;
[0037] S3. Centrifugally filter the mixed slurry obtained in step S2 to obtain filter residue 1 and filtrate 1. Clean filter residue 1 with pure water for 1-2 times and then centrifugally filter to obtain filter residue 2 and filtrate 2. Dry filter residue 2 to obtain high-purity 4-cyanopyridi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com