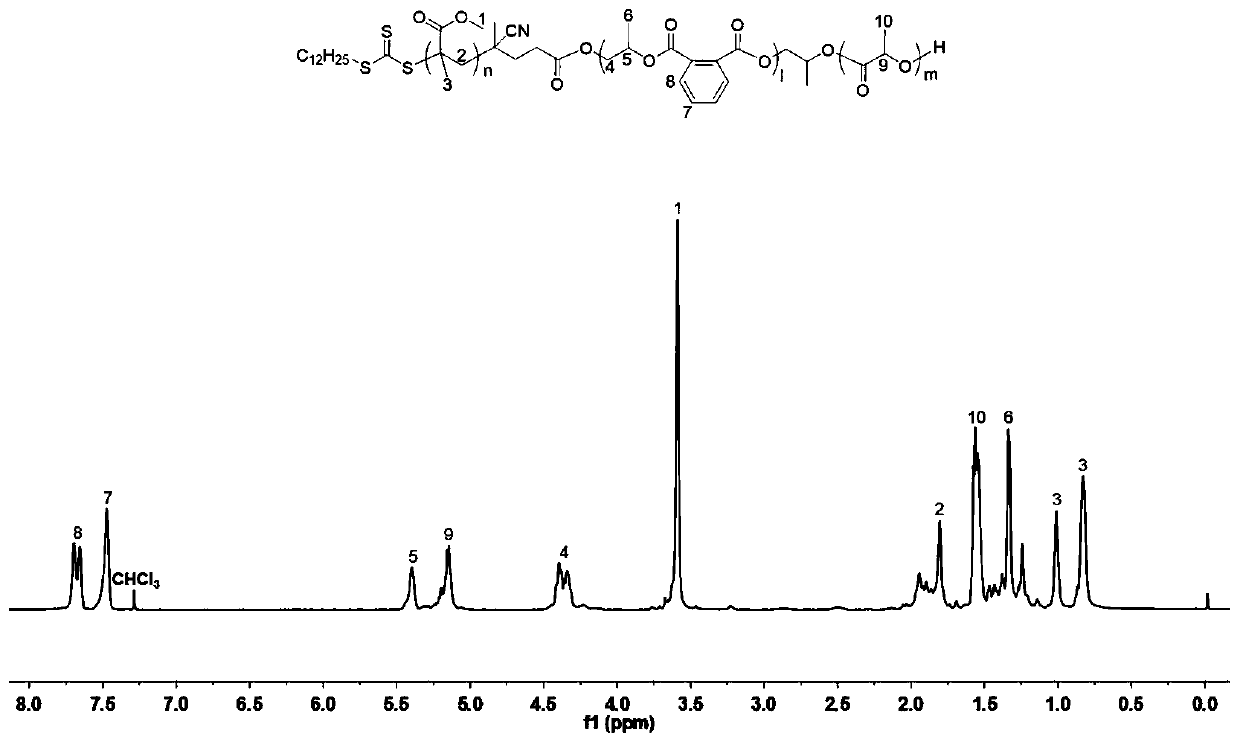
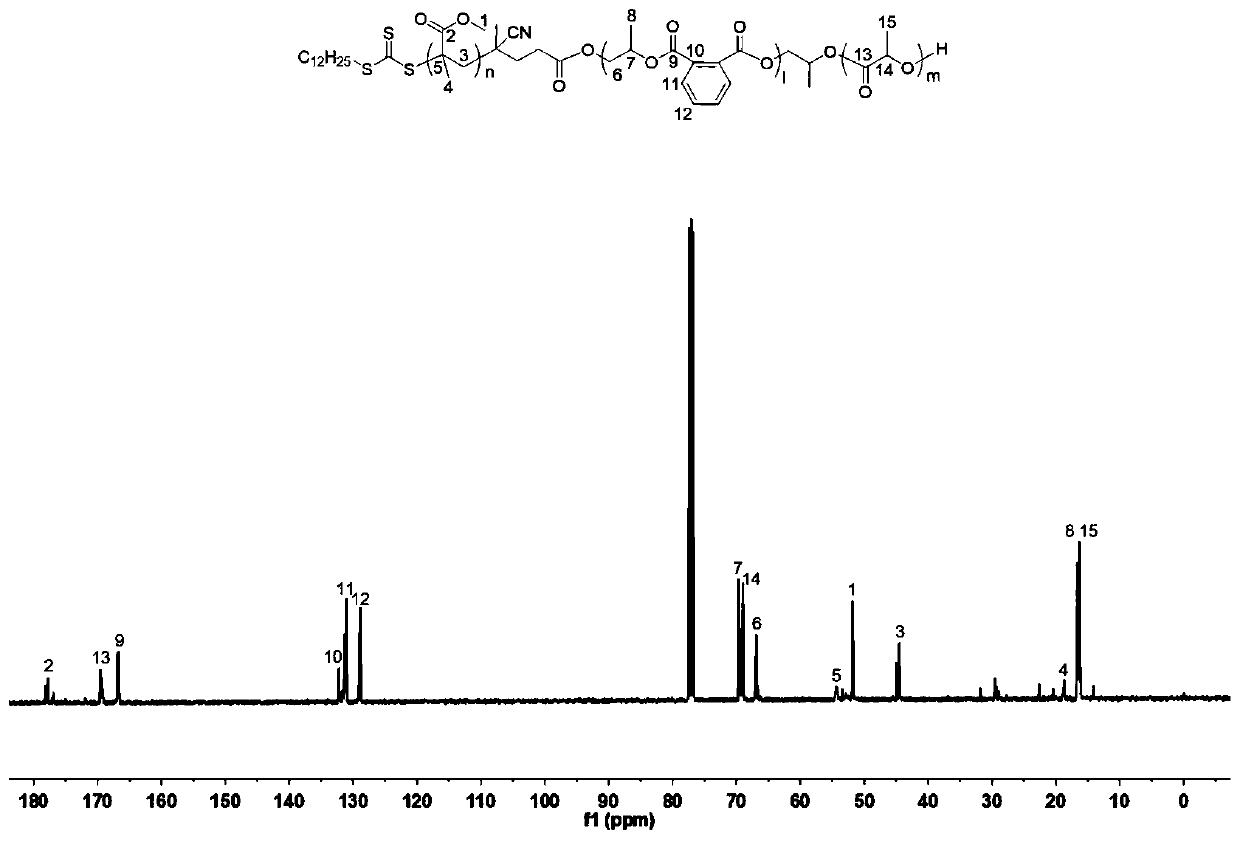
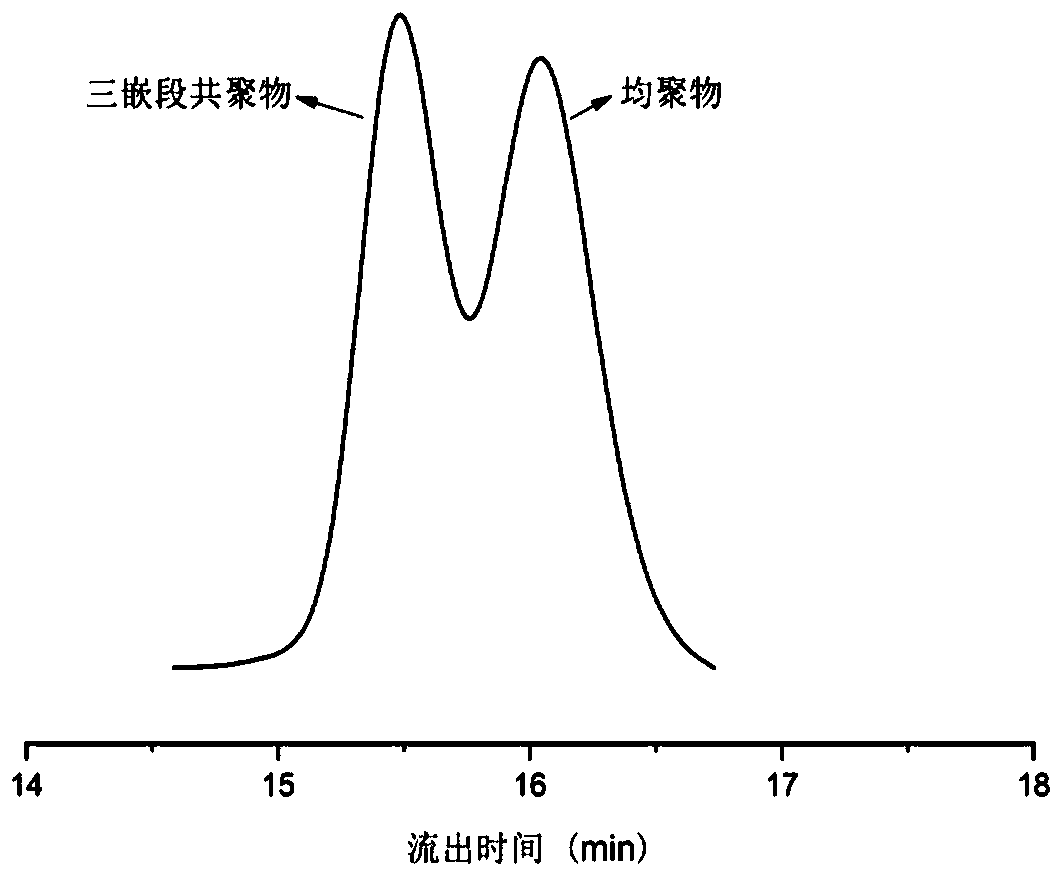
Preparation method of polyacrylate-polyester I-polyester II triblock copolymer
A technology of polyacrylates and acrylates, which is applied in the field of preparation of polyacrylate-polyester I-polyester II triblock copolymers, can solve problems such as harsh reaction conditions, achieve easy operation, improve performance, and structure Various types of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] In some embodiments, the preparation method of the present invention includes the following steps:
[0044] (1) The monomer mixed solution is mixed with a multifunctional chain transfer reagent, a catalyst and a free radical initiator to obtain a pre-reaction mixed solution; the monomer mixed solution is an acrylic ester monomer, an epoxy monomer, an acid anhydride A mixed solution of monomers and lactone monomers;
[0045] (2) reacting the pre-reaction mixed solution at 50-80° C. for 12-48 hours to obtain a reaction mixture;
[0046] (3) cooling the reaction mixture described in step (2) to room temperature (20-30° C.), quenching the reaction by introducing air, dissolving the reaction mixture with a polar organic solvent to obtain a mixed solution containing a block copolymer, Add the mixed solution containing the block copolymer dropwise into the precipitating agent to precipitate, filter and separate the precipitate, and dry to obtain the polyacrylate-polyester I-p...
Embodiment 1
[0084] A preparation of polyacrylate-polyester I-polyester II triblock copolymer, under anhydrous and oxygen-free conditions, methyl methacrylate, 1,2-epoxybutylene, ε-caprolactone, After phthalic anhydride and multifunctional chain transfer reagent CTA-1 are mixed, triethyl boron and 1,5,7-triazide bicyclo (4.4.0) dec-5-ene and free radical initiator A one-step polymerization reaction under the combined action of azobisisobutyronitrile. Among them, CTA-1 is a carboxy-terminal trithioester compound with the following structure:
[0085]
[0086] The preparation of this polyacrylate-polyester I-polyester II triblock copolymer specifically comprises the following steps:
[0087] (1) Under anhydrous and anaerobic conditions, methyl methacrylate, 1,2-epoxybutylene, ε-caprolactone, phthalic anhydride and multifunctional chain transfer reagent CTA-1 are mixed according to 250: Take 2.6ml, 4.1mL, 0.5mL, 0.74g, and 80mg at a molar ratio of 500:50:50:2, respectively, and add them ...
Embodiment 2
[0092] A kind of preparation of polyacrylate-polyester I-polyester II triblock copolymer, under anhydrous and oxygen-free condition, benzyl methacrylate, propylene oxide, lactide, norbornene diacid anhydride and After the multifunctional chain transfer reagent CTA-2 is mixed, the composite catalyst triethylboron and 7-methyl-1,5,7-triazabicyclo (4.4.0) dec-5-ene and the free radical initiator couple A one-step polymerization reaction under the combined action of nitrogen diisoheptanonitrile. Among them, CTA-2 is a carboxyl-terminal trithioester compound with the following structure:
[0093]
[0094] The preparation of this polyacrylate-polyester I-polyester II triblock copolymer specifically comprises the following steps:
[0095] (1) Under anhydrous and oxygen-free conditions, benzyl methacrylate, propylene oxide, lactide, norbornene dioic anhydride and multifunctional chain transfer reagent CTA-2 according to 50:500:150:150: Take 0.85mL, 3.5mL, 2.16g, 2.46g, and 0.36g ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com