Synthesis of 1,1-dibromo-2,2-bis(chloromethyl)cyclopropane
A technology of chloromethyl propane and a synthesis method, which is applied in the field of synthesis of 1,1-dibromo-2,2-bicyclopropane, can solve the problems of harsh conditions, difficulty in amplification and high cost, and achieves improved yield and reduced instantaneous Heat release, the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
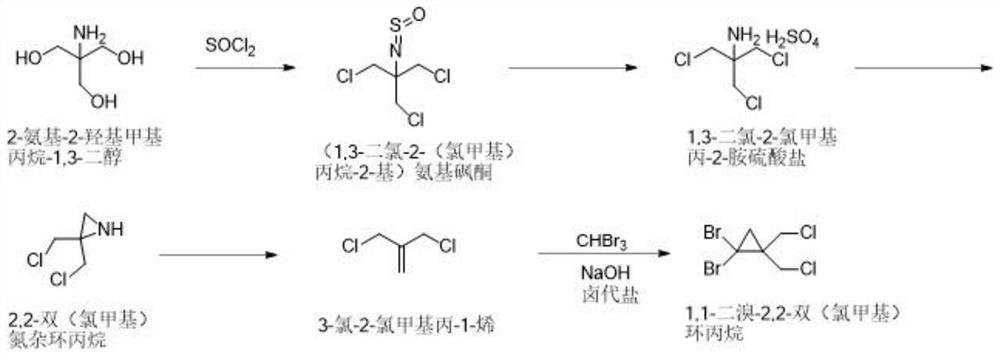
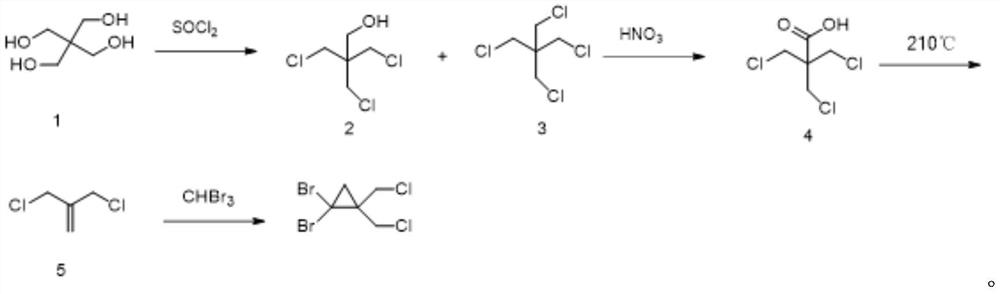
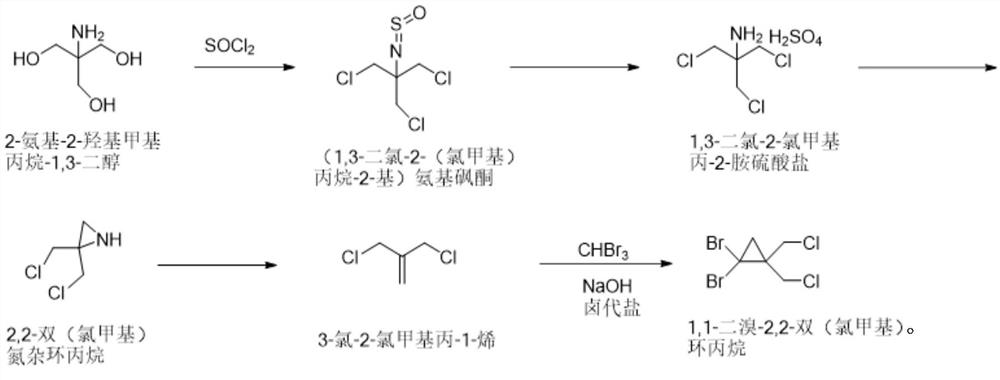
[0037] The synthetic method of 1,1-dibromo-2,2-bis(chloromethyl)cyclopropane of the present invention comprises the following steps: 2-amino-2-hydroxymethylpropane-1,3-diol and thionyl chloride Chlorine substitution reaction to obtain (1,3-dichloro-2-(chloromethyl)propan-2-yl) aminosulfone ketone; then hydrolysis by concentrated sulfuric acid aqueous solution to obtain 1,3-dichloro-2-chloromethyl Propan-2-amine sulfate; followed by salt solution under alkaline conditions, and ring closure to obtain 2,2-bis(chloromethyl)aziridine; then react with sodium nitrite to obtain 3-chloro-2-chloro Methylprop-1-ene; Finally, it reacts with tribromomethane under basic conditions to generate 1,1-dibromo-2,2-bis(chloromethyl)cyclopropane. In the present invention, 1,1-dibromo-2,2-bis(chloro Methyl) cyclopropane, the reaction formula that synthetic method involves is as figure 1 shown.
Embodiment 2
[0039] Based on embodiment 1, the specific synthetic method of the present invention is as follows:
[0040] Synthesis of step A, (1,3-dichloro-2-(chloromethyl)propane-2-yl)aminosulfone ketone: 5kg2-amino-2-hydroxymethylpropane-1,3-diol is dissolved in In toluene, add 25 kg of thionyl chloride under temperature control at 20-40 ° C, add 0.653 kg of pyridine after cooling down to 10-15 ° C, react at room temperature for 2 hours, and gradually increase the temperature to 105-110 ° C for 10 hours; after the reaction, 9.18 Toluene solution of kg (1,3-dichloro-2-(chloromethyl)propan-2-yl)aminosulfone, yield 100%.
[0041] Synthesis of step B, 1,3-dichloro-2-chloromethylpropan-2-amine sulfate: 9.18kg (1,3-dichloro-2-(chloromethyl)propane-2- Base) the toluene solution of aminosulfone ketone is controlled at a temperature not exceeding 25°C, and water and concentrated sulfuric acid aqueous solution are added; the temperature is raised to 85-95°C and reacted for 15-22 hours; , the aq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com