Fluorescent probe for detecting mitochondrial membrane potentials, and preparation method and application thereof
A technology of mitochondrial membrane potential and fluorescent probes, which is applied in the field of analytical chemistry, can solve the problems of single species, and achieve the effects of low biological toxicity, simple purification steps, and simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
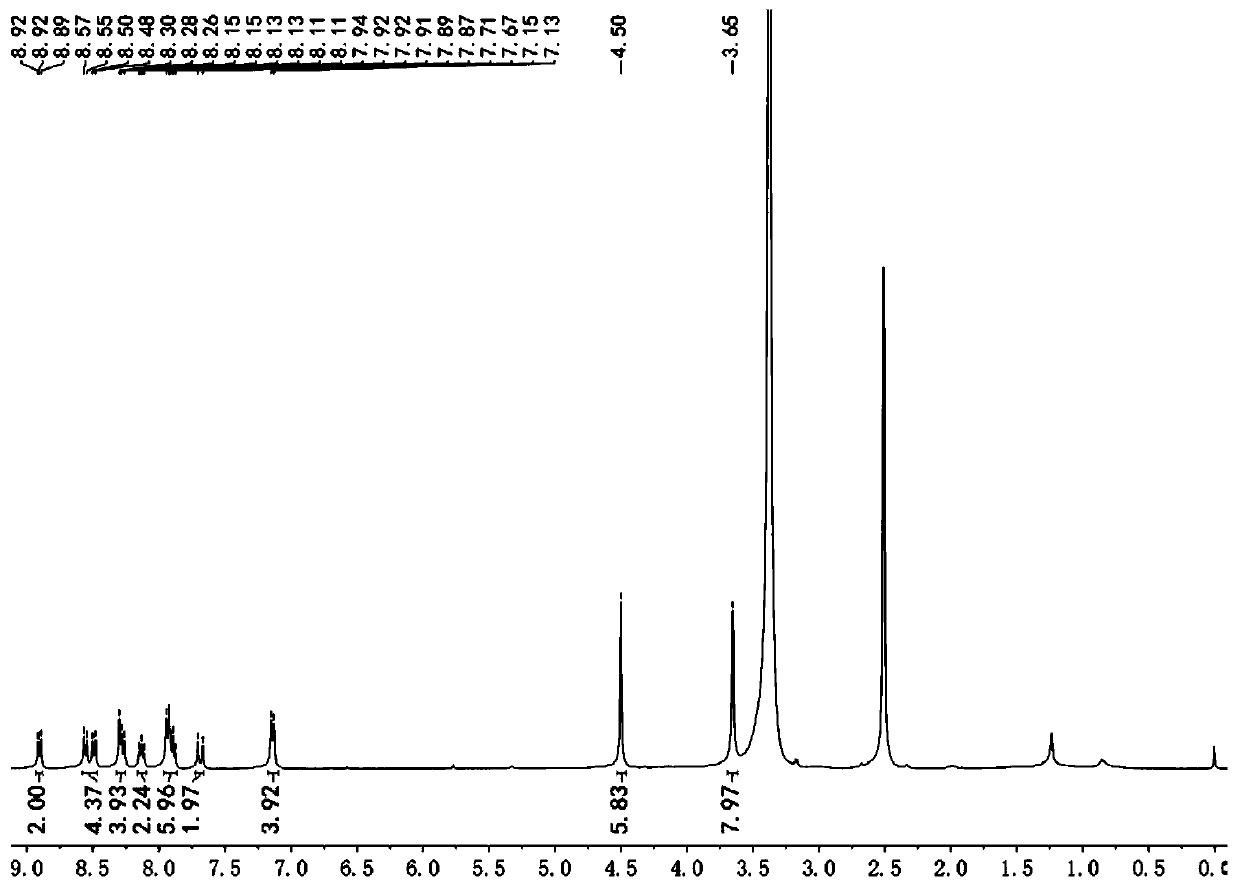
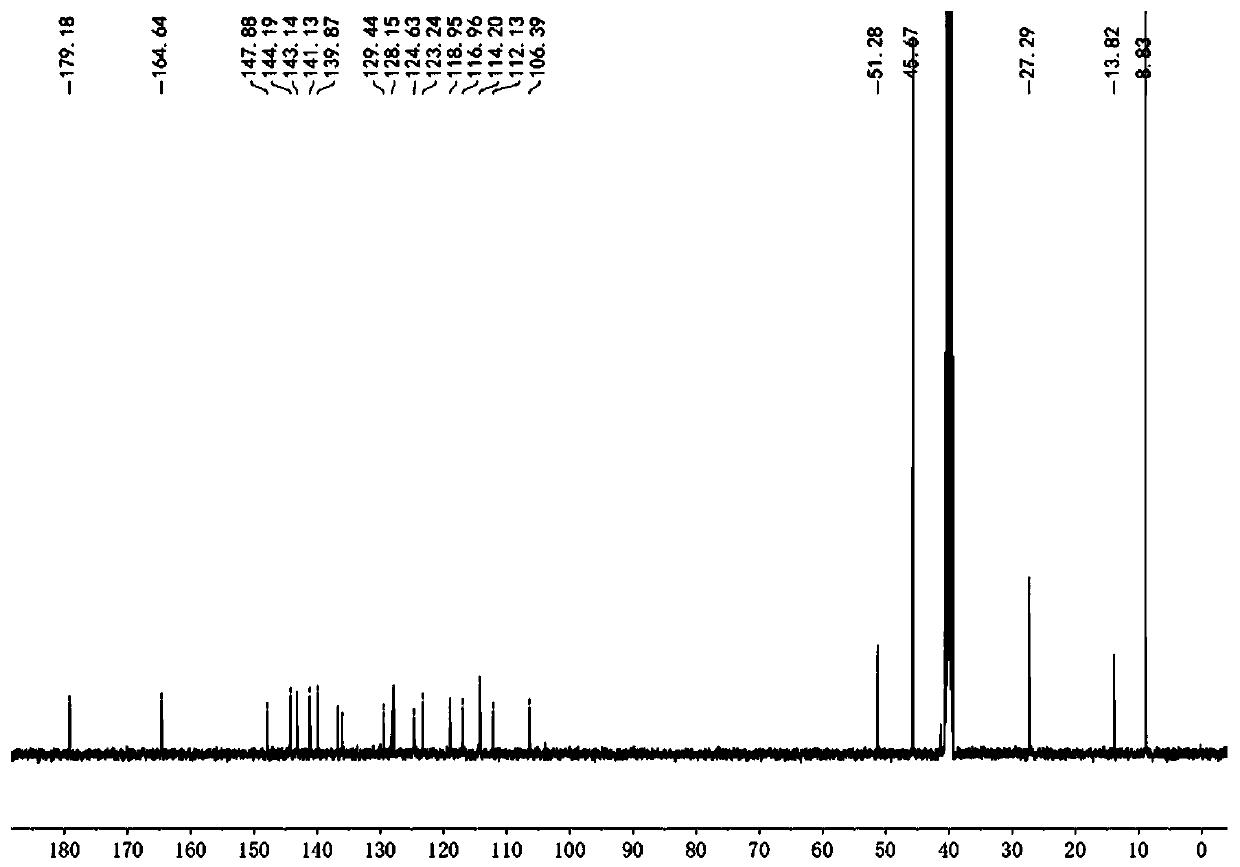
[0036] Example 1 Synthesis of Fluorescent Probes
[0037] (1) Synthesis of 1,2-dimethyl-quinoline iodide salt (compound 1):
[0038] Add 10 mL of ethanol to the round bottom flask, then add 1.1 mL of 2-methylquinoline, add 0.5 mL of iodomethane, heat to 60 °C for 30 hours, after the reaction is completed, cool the reaction system to room temperature, and a solid precipitates , filtered and washed with ethanol to give 1,2-dimethyl-quinoline iodide salt (compound 2) with a yield of 92%. 1 H NMR (400 MHz, DMSO-d6) δ9.12 (d, J = 8.5 Hz, 1H), 8.60 (d, J = 9.0 Hz, 1H), 8.41 (dd, J = 8.2, 1.6Hz, 1H), 8.24 (ddd, J = 8.8, 7.0, 1.6 Hz, 1H), 8.14 (d, J = 8.5 Hz, 1H), 8.00 (t, J = 7.6 Hz, 1H), 4.45 (s, 3H), 3.09 (s , 3H).
[0039] (2) Synthesis of 4,4'-(piperazine-1,4-diyl)benzaldehyde (compound 2):
[0040] Piperazine (1.0 g, 11.62 mmol) was dissolved in H 2 O (18 mL) and 2-methoxyethanol (20 mL). The mixture was heated to reflux, then a solution of 4-fluorobenzaldehyde (0.63 mL, 5...
Embodiment 2
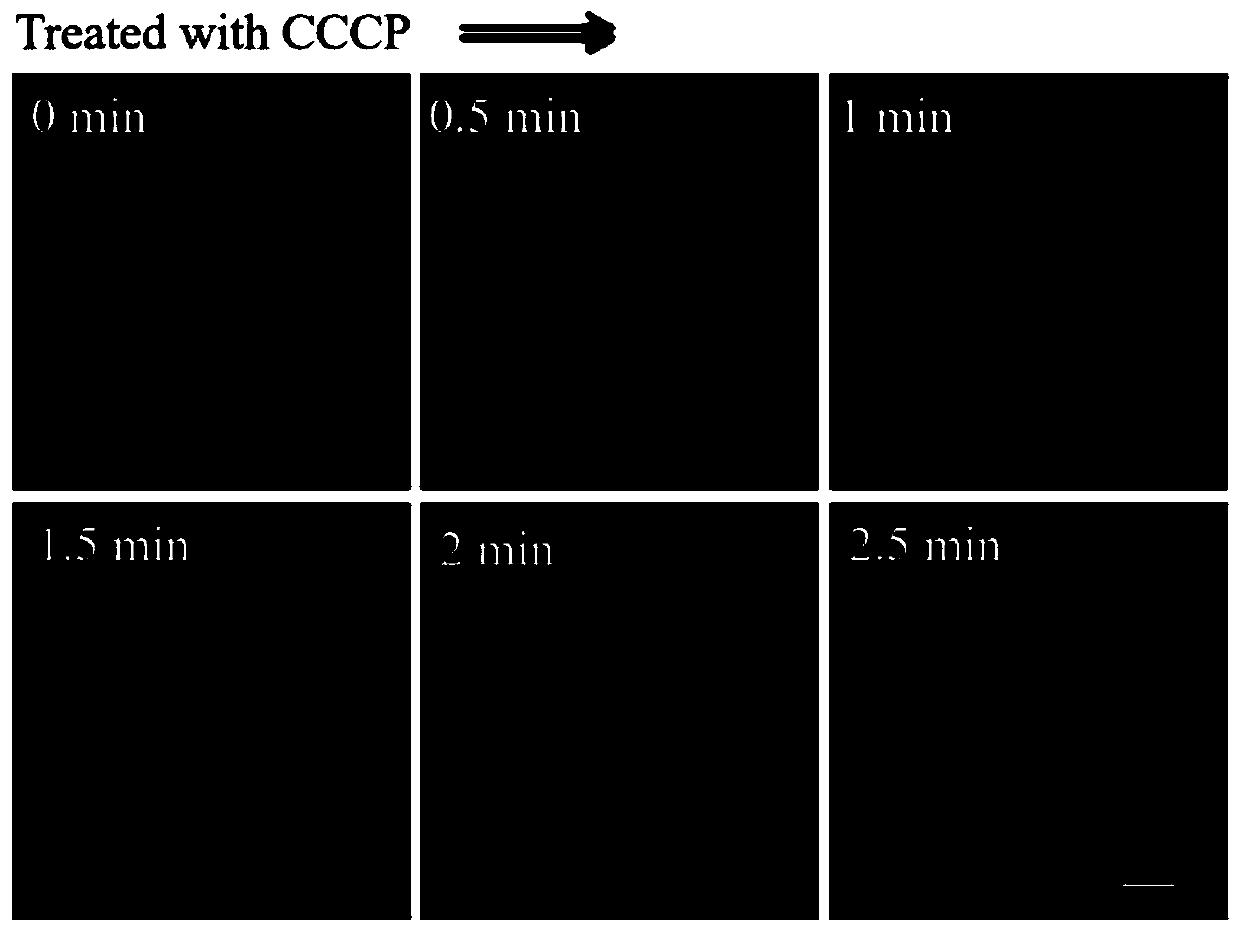
[0043] Example 2 Responses of fluorescent probes to different membrane potentials
[0044] Set the density to 3 x 10 5 cells / mL of HeLa cells were inoculated into sterile 35 mm imaging culture dishes in CO 2 Incubator (at 37°C, 5% CO 2 ) for more than 12 hours to allow the cells to adhere to the wall. Then prepare the DMSO solution of the probe HJI obtained in Example 1 with a concentration of 1 mM as the mother solution, add the mother solution to the cell culture dish so that the final concentration is 5 μM, continue to cultivate for 20 min, and then absorb the cell culture solution Go, wash the cells with PBS buffer 3 times, add 1 mL of fresh medium, and then add 10 μM CCCP (an oxidative phosphorylation uncoupler that can reduce the mitochondrial membrane potential) for imaging immediately, every 0.5 minutes, record different Imaging pictures under time.
[0045] In the cell imaging experiment, the excitation wavelength is 488 nm, and the detection band is the red ligh...
Embodiment 3
[0046] Example 3 Selectivity of fluorescent probes to different ions
[0047] Configure the DMSO mother solution of the fluorescent probe prepared in Example 1, the concentration is 5 mM, prepare different amino acids (Ile, Arg, Ser, Asn, Gln, Glu, His, Ala, Hcy, N-Ace, Val, GSH) and NaCl, KNO 3 、H 2 o 2 stock solution in PBS at a concentration of 100 mM. Then, 5 μL of the probe mother solution was added to 5 mL volumetric flasks, 10 μL of different analytes were added to each volumetric flask, and finally the volume was adjusted to 5 mL with PBS buffer solution. Fluorescence detection (excitation wavelength 500 nm) was then performed. Take the wavelength as the abscissa and the fluorescence intensity as the ordinate Figure 4 . It can be seen from the figure that the addition of different analytes has no effect on the fluorescence of the probe.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com