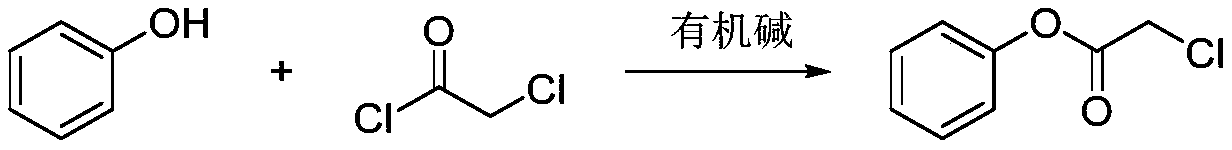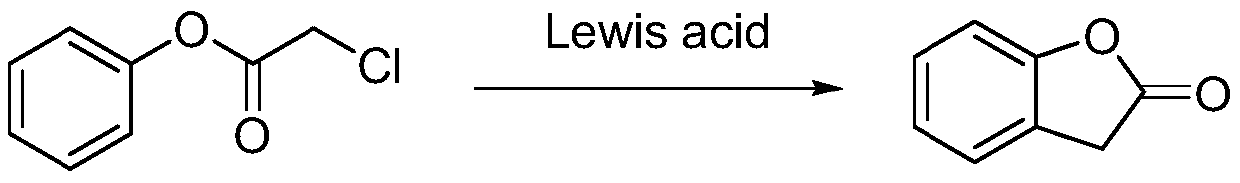Preparation method of benzofuran-2-(3H)-ketone
A technology for benzofuran and phenol, applied in the field of preparation of benzofuran-2-one, can solve the problems of large amount of solvent, cumbersome post-processing process, unenvironmental process, etc., and achieves simple and easy-to-obtain raw materials and stable catalyst structure. , the effect of high industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The first step, synthesis of α-chloroacetic acid phenol ester
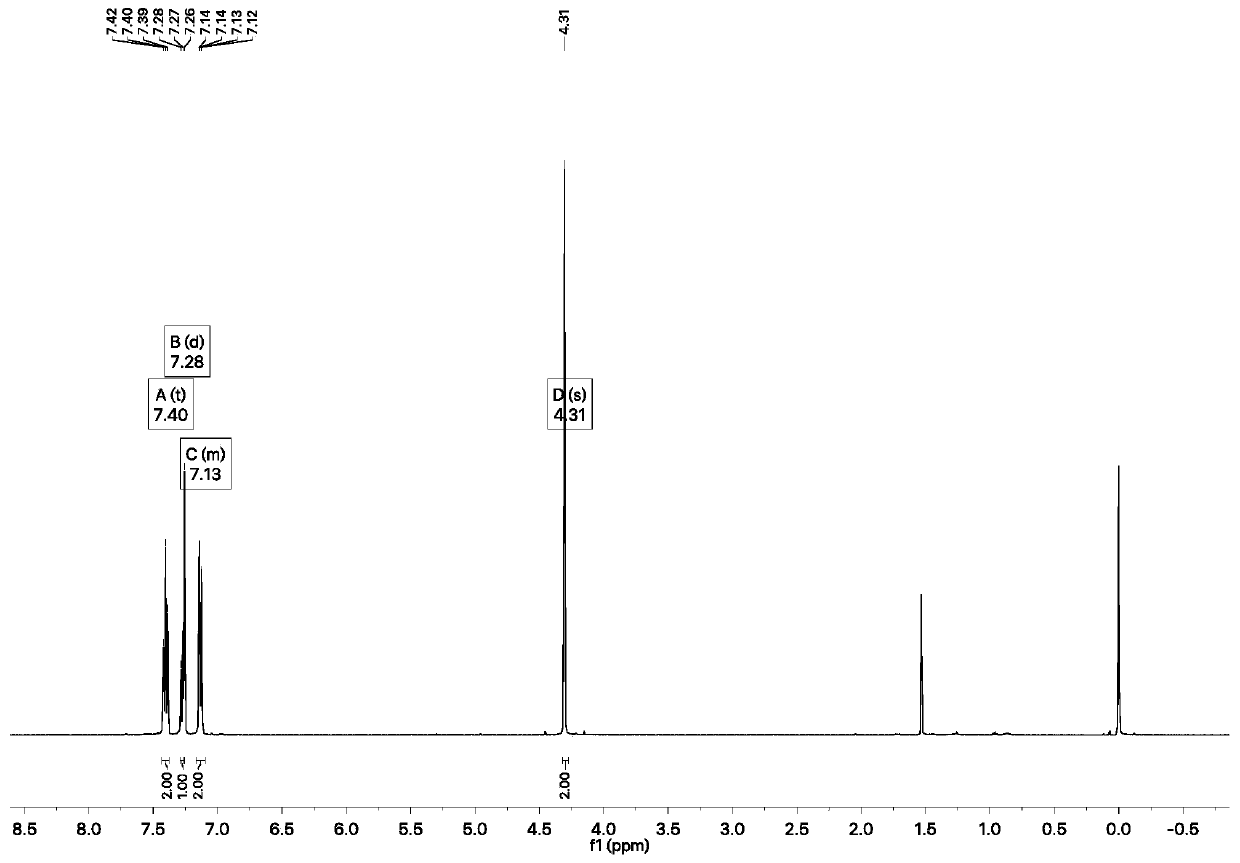
[0023] Weigh 2.00g of phenol and 2.40g of chloroacetyl chloride and add them into 50mL of dichloromethane, where the concentration of phenol is 0.5mol / L, then add 2.14g of triethylamine, cool down to -20°C, and react for 1h. After the end, add acid and Washed with saturated brine and post-treated to obtain 2.56 g of white solid with a yield of 70%. α-Chloroacetyl chloride 1 H-NMR spectrum as figure 1 as shown, 1 H NMR (500MHz, Chloroform-d) δ7.40(t, J=7.8Hz, 2H), 7.28(d, J=7.8Hz, 1H), 7.16–7.09(m, 2H), 4.31(s, 2H) .
[0024] The second step, synthesis of benzofuran-2-(3H)-one
[0025] Add 1.00g of α-phenol chloroacetate generated in the first step into 10ml of carbon tetrachloride, and dissolve in 0.04g of anhydrous AlCl 3 The Friedel-Crafts reaction occurred under the condition of 20° C., the reaction time was 3 h, and the yield was 83% as detected by TLC.
Embodiment 2
[0027] The first step, synthesis of α-chloroacetic acid phenol ester
[0028] Weigh 2.00g of phenol and 4.81g of chloroacetyl chloride and add them to 50mL of carbon tetrachloride, where the concentration of phenol is 1.0mol / L, add 4.73g of sodium phenoxide, cool down to 0°C, and react for 6h, then add acid and saturated salt After washing with water, 2.72 g of white solid was obtained after treatment, with a yield of 75.21%. α-Chloroacetyl chloride 1 H-NMR spectrum as figure 1 shown.
[0029] The second step, synthesis of benzofuran-2-(3H)-one
[0030] Add 1.00g of α-phenol chloroacetate generated in the first step into 10ml of dichloromethane, and react under the condition of 0.017g of p-toluenesulfonic acid, the reaction temperature is 60°C, and the reaction time is 8h. After TLC detection, the product The rate is 73.6%.
Embodiment 3
[0032] The first step, synthesis of α-chloroacetic acid phenol ester
[0033] Weigh 2.00g of phenol and 7.21g of chloroacetyl chloride and add them into 50mL of chloroform, where the concentration of phenol is 1.5mol / L, add 3.45g of sodium carbonate, heat up to 60°C, react for 4h, add acid and saturated saline to wash after completion, After working up, 2.64 g of white solid were obtained, the yield was 72.88%. α-Chloroacetyl chloride 1 H-NMR spectrum as figure 1 shown.
[0034] The second step, synthesis of benzofuran-2-(3H)-one
[0035] Add 1.00g of α-phenol chloroacetate generated in the first step into 10ml of dichloromethane, and a Friedel-Crafts reaction occurs under the condition of 0.16g of anhydrous ferric chloride, the reaction temperature is 80°C, and the reaction time is 12h. As detected by TLC, the yield was 78.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com