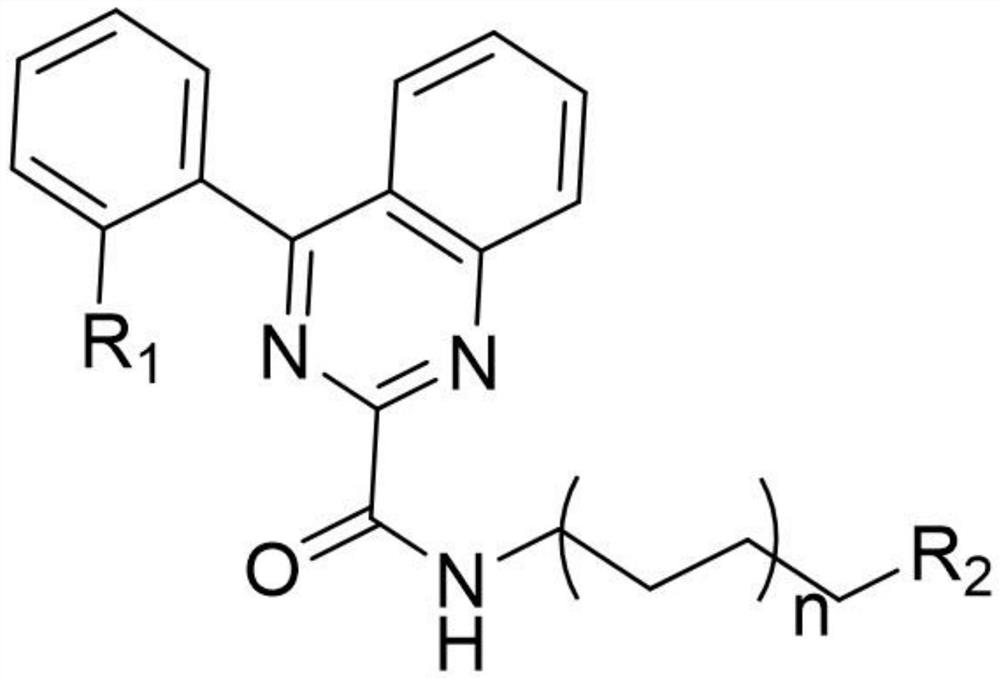
A kind of quinazoline fluorescent probe and its preparation method and application
A fluorescent probe, quinazoline technology, applied in the field of biomedicine, can solve the problems of large autofluorescence background interference, high price and popularization, affecting the detection effect, etc., achieves important application value, avoids autofluorescence interference, and has good affinity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
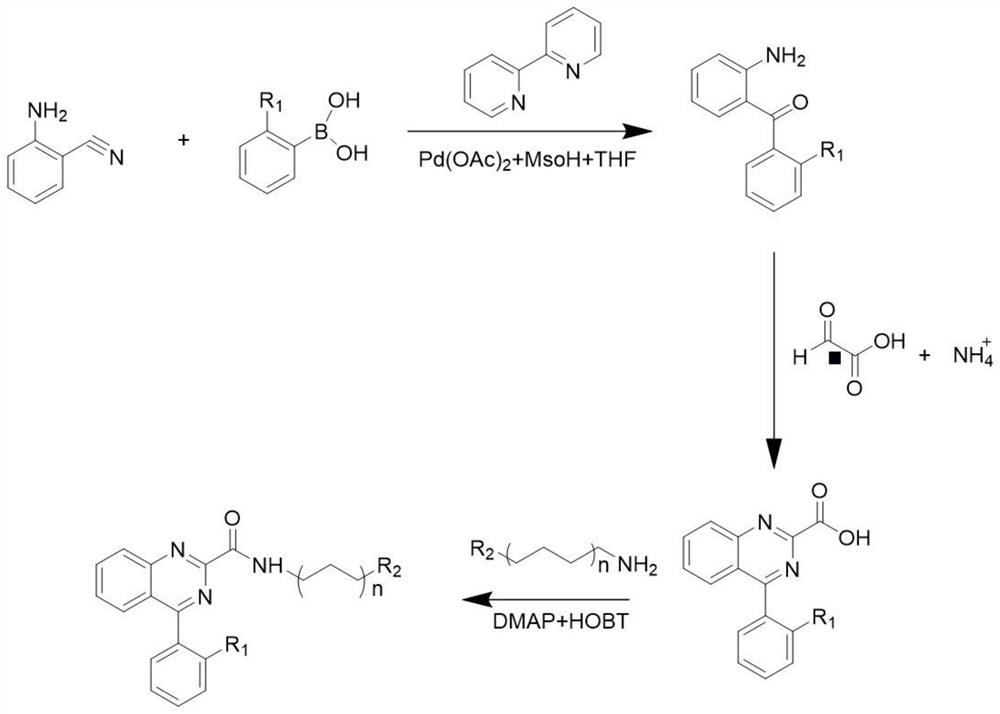
[0050] The embodiment of the present application provides the synthesis method of quinazoline fluorescent probe, and the specific steps are as follows:
[0051] According to the synthetic route of reactant 1 Synthetic Reactant 1:
[0052] 4.3g, 31.35mmol of (N,N-Dimethyl-m-aminophenol) was added to a mixed solution of 25ml of concentrated hydrochloric acid and 10ml of water, the temperature was kept at -5°C, and 20ml of sodium nitrite (2.18g, 31.60mmol) aqueous solution was added slowly, mechanically Stir for 2h, let it stand, and filter with suction to obtain a brown-yellow solid. The crude product is washed with 50ml of saturated sodium acetate solution to obtain reactant 1 (4.20g, 81%) of dark red solid powder, and the structural formula of reactant 1 is
[0053] According to the synthetic route of reactant 2 Synthetic Reactant 2:
[0054] 4.12g, 19.90mmol of (α-bromonaphthalene) and 4.65g, 40.02mmol of (1,6-hexanediamine) was added to 40ml of ethylene glycol ...
Embodiment 2
[0067] In the embodiment of the present application, the product 1 is subjected to spectral determination, and the specific steps are as follows:
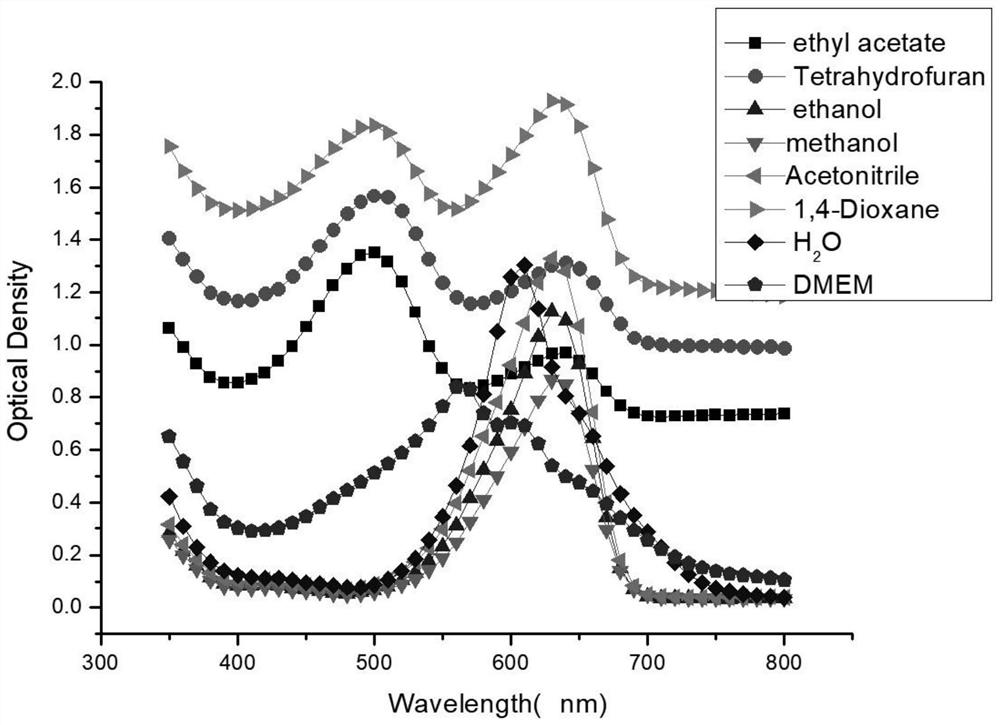
[0068] The product 1 prepared in Example 1 was made into different concentrations (respectively 200 μM, 100 μM, 50 μM, 25 μM, 12.5 μM and 0 μM) and dissolved in ethyl acetate, tetrahydrofuran, ethanol, methanol, acetonitrile, 1,4-bis In oxane, water or DMEM, use a microplate reader to scan the absorption spectrum at all wavelengths to find the maximum absorption wavelength, then use the maximum absorption wavelength as the excitation wavelength, and measure the maximum emission wavelength. The results are as follows Figure 3-4 as shown, image 3 It is the absorption spectrum figure of the product 1 of the embodiment of the present application in different solvents, Figure 4 It is the emission spectrum diagram of the product 1 in the embodiment of the present application in different solvents. It can be seen that the maximum emis...
Embodiment 3
[0070] In the embodiment of the present application, the product 1 was subjected to a cytotoxicity experiment, and the specific steps were as follows:
[0071] In this experiment, the thiazolium blue MTT method was used to evaluate the toxicity of product 1 to cells by the survival rate of cells. BV2 cells were divided into 5×10 4 Seed in a 96-well plate at a density of / ml, add 100 μL of cell solution to each well, and after 24 hours, the cells adhered to the wall, and the supernatant was sucked away, and the product 1 was dissolved in DMEM, and the concentration was divided into 200 μM, 100 μM, 50 μM, 25 μM, Incubate at 5 μM and 3 μM, add 100 μL of DMEM solution of product 1 to each well, absorb the supernatant after 24 hours, add 100 μL of MTT solution (500 μg / ml) to each well, continue to incubate for 4 hours, and measure the concentration at 570 nm on a microplate reader. OD value. Cell survival rate=(OD experimental group-OD blank group) / (OD control group-OD blank grou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com