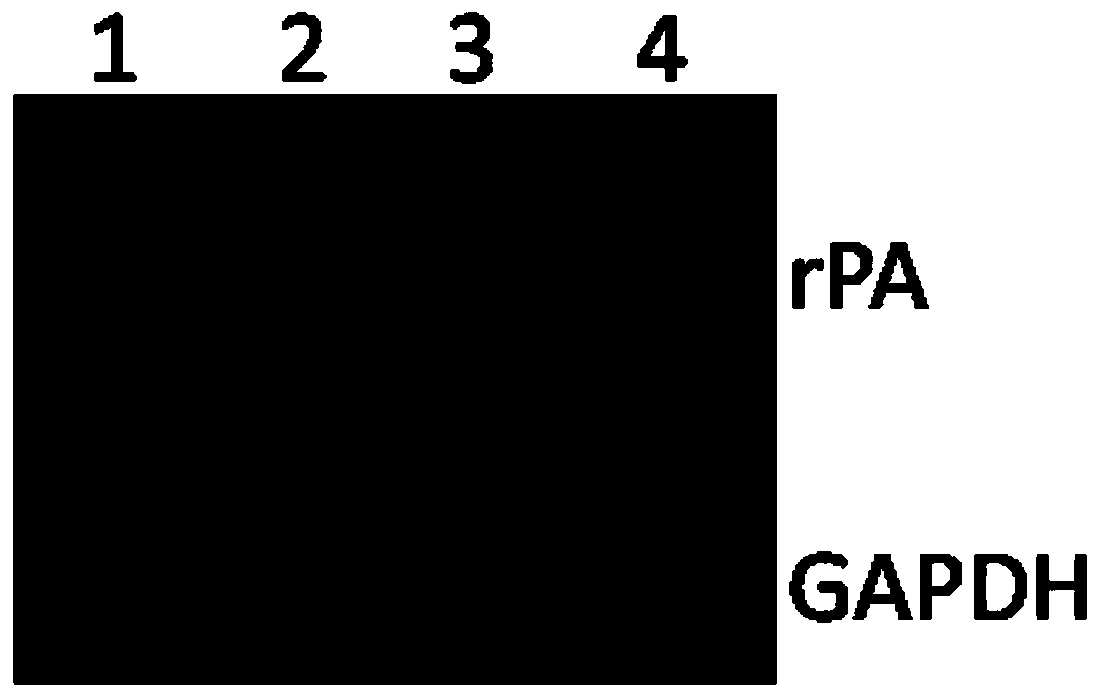
Cell strain and method of expressing reteplase (rPA) by human source cells
A technology of reteplase and cell line, applied in the field of biopharmaceuticals, can solve the problems of low yield, high cost and long cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Construction of rPA lentiviral expression plasmid
[0061] 1. The complete DNA sequence of rPA was obtained through literature search and analysis, and the DNA sequence (SEQ ID No.2) encoding rPA was obtained by in vitro total gene synthesis by chemical synthesis, and EcoRI (CAS : 1040S, Treasure Bioengineering Co., Ltd.) and NotI enzyme (CAS: 1166S, Treasure Bioengineering Co., Ltd.) sites, and then rPA was recombined into the lentiviral expression vector plenti-puro (CAS: 39481, Addgene ), the correct rPA expression plasmid plenti-rPA-puro was obtained by sequencing.
Embodiment 2
[0063] Construction of rPA expressing cell line
[0064] 1. Preparation of lentiviral particles expressing rPA.
[0065] (1) Pack lentiviral particles according to the 3-plasmid system (pMD2.G, psPAX2, plenti-rPA-puro) (Addgene), and use the 3 plasmids according to a specific ratio (pMD2.G:psPAX2:plenti-rPA-puro=5: 10:15 μg), introduced into 10 by lipofection method (CAS: 40802ES02, Shanghai Yisheng Biotechnology Co., Ltd.) 6 human-derived 293T cells (ATCC), and then put the 293T cells in CO 2 Cultivated in an incubator;
[0066] (2) After culturing for 48 hours, the culture supernatant was collected by centrifugation, 1200 rpm, 5 minutes, an equal amount of culture medium was added to the cells, and the culture was continued;
[0067] (3) After continuing to cultivate for 24 hours, collect the culture supernatant by centrifugation, 1200rpm, 5min;
[0068] (4) The cell culture supernatant collected twice was combined, and 10% volume of PEG8000 / NaCL solution (CAS: A100159-0...
Embodiment 3
[0095] rPA thrombolytic activity assay
[0096] 1. Preparation of fibrin plate: Weigh 0.02g of fibrin (CAS: 9001-32-5, Sigma-Aldrich), add 5ml of PBS, mix and place in a 37°C water bath to preheat until fully dissolved, and take another appropriate amount of coagulation Enzyme (CAS: 9002-04-4, Sigma-Aldrich) was added to 1ml of PBS solution, mixed gently, and placed in a 37°C water bath to preheat; 0.07g of agarose (CAS: 9012-36-6, Sigma-Aldrich) was weighed -Aldrich), add 7ml of PBS solution, heat to dissolve and then cool to about 50°C to obtain an agarose solution; mix the prepared thrombin solution and fibrin solution and immediately add it to the agarose solution, and pour it into the 10cm diameter plates, cooled at room temperature to obtain fibrin plates.
[0097] 2. Use a pipette tip to punch holes in the fibrin plate prepared in Step 1, add 5 μl of the rPA sample prepared in Example 2, and then place it in a 37° C. incubator for 12 hours and observe the size of the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com