Method for excreting and expressing recombinant human granulocyte-colony factor in colon bacillus
A granulocyte and colony technology, applied in the direction of recombinant DNA technology, chemical instruments and methods, botany equipment and methods, etc., can solve the problem that foreign proteins cannot be glycosylated, affect the formation of correct conformation, and are unfavorable for large-scale cultivation and purification And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
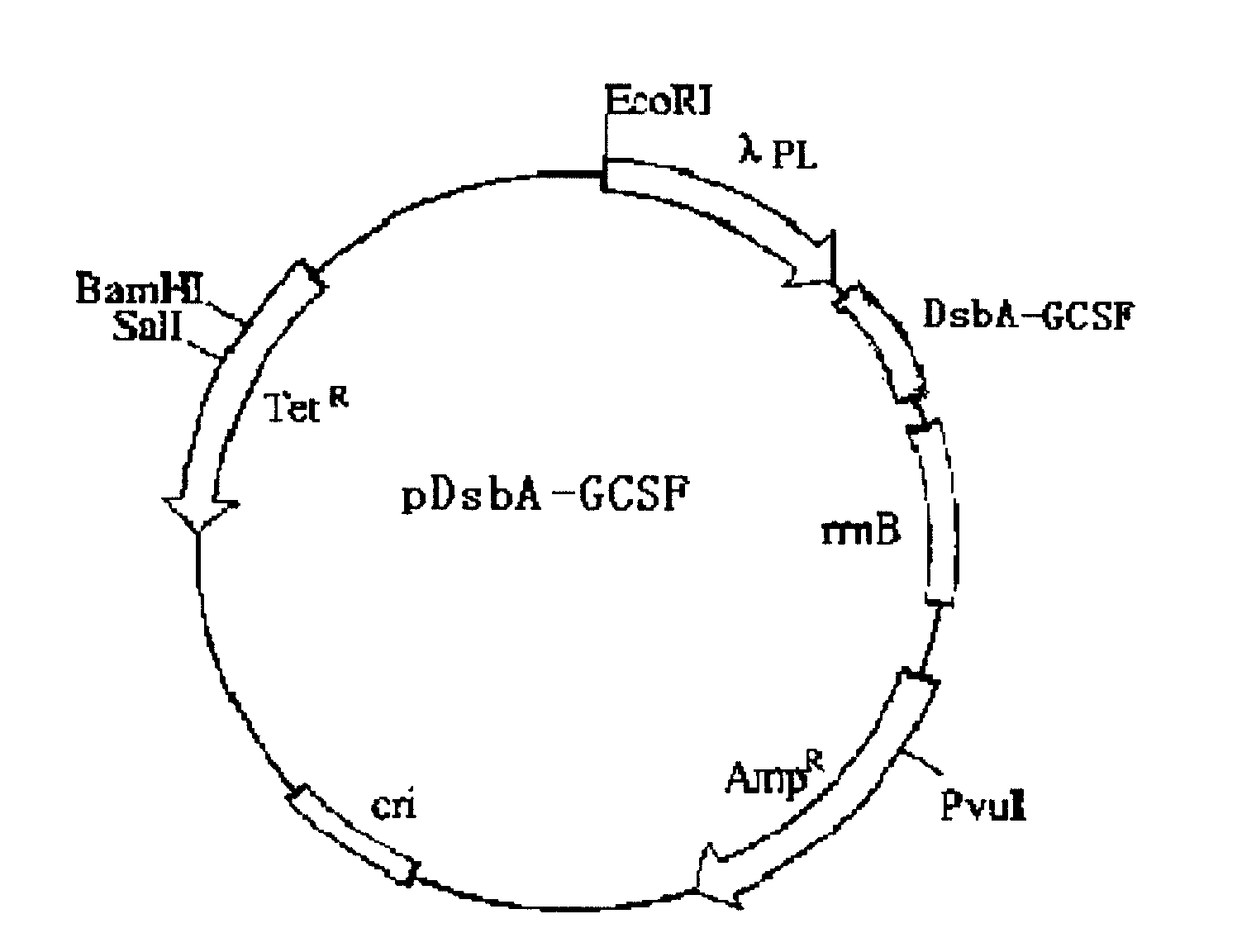
[0021] The sequence of λPL promoter and PCR primers for synthesis are cloned from Escherichia coli vector pBLV by PCR method, and PCR amplification is carried out using it as a template. The upstream primer used was: 5'GTGAATTCAGATCTCTCACCTACCAAAC3' (containing EcoRI), and the downstream primer was: 5'GTCATATGACTAGTCCTCCTTAATTTTTAACCAATG 3' (containing the Spel site). After the PCR product was digested by EcoRI / Spel double enzymes, it was recovered by low-melting gel dispensing for future use.
Embodiment 2
[0023] The human GCSF gene is already available in our company. The designed primers are obtained by PCR from the original preserved plasmid. Two extension primers are designed upstream. Primer 1: 5'-GGT TTA GTT TTA GCG TTT AGC GCA TCG GCGACACCATTAGGCCCTGCCAGC-3', Primer 2: 5 '-GAGGAATTCATG AAA AAG ATT TGG CTGGCG CTG GCT GGT TTA GTT TTA GCG TTT AGC-3' downstream primer 5'-GCGGGATCCTCACGGCTGGGCAAG-3', the amplified fragment is about 600bp in length. The second GCA at the 5' end is connected to the coding DNA of the last amino acid of the DsbA signal peptide, so that the first amino acid of the human GCSF mature protein is (Thr), so the amino terminal of GCSF secreted after the signal peptide is excised does not contain Met, And consistent with natural GCSF. The amino acid sequence of the signal peptide is: Met Lys Lys Ile Trp Leu Ala Leu Ala Gly Leu Val Leu Ala Phe Ser Ala SerAla
Embodiment 3
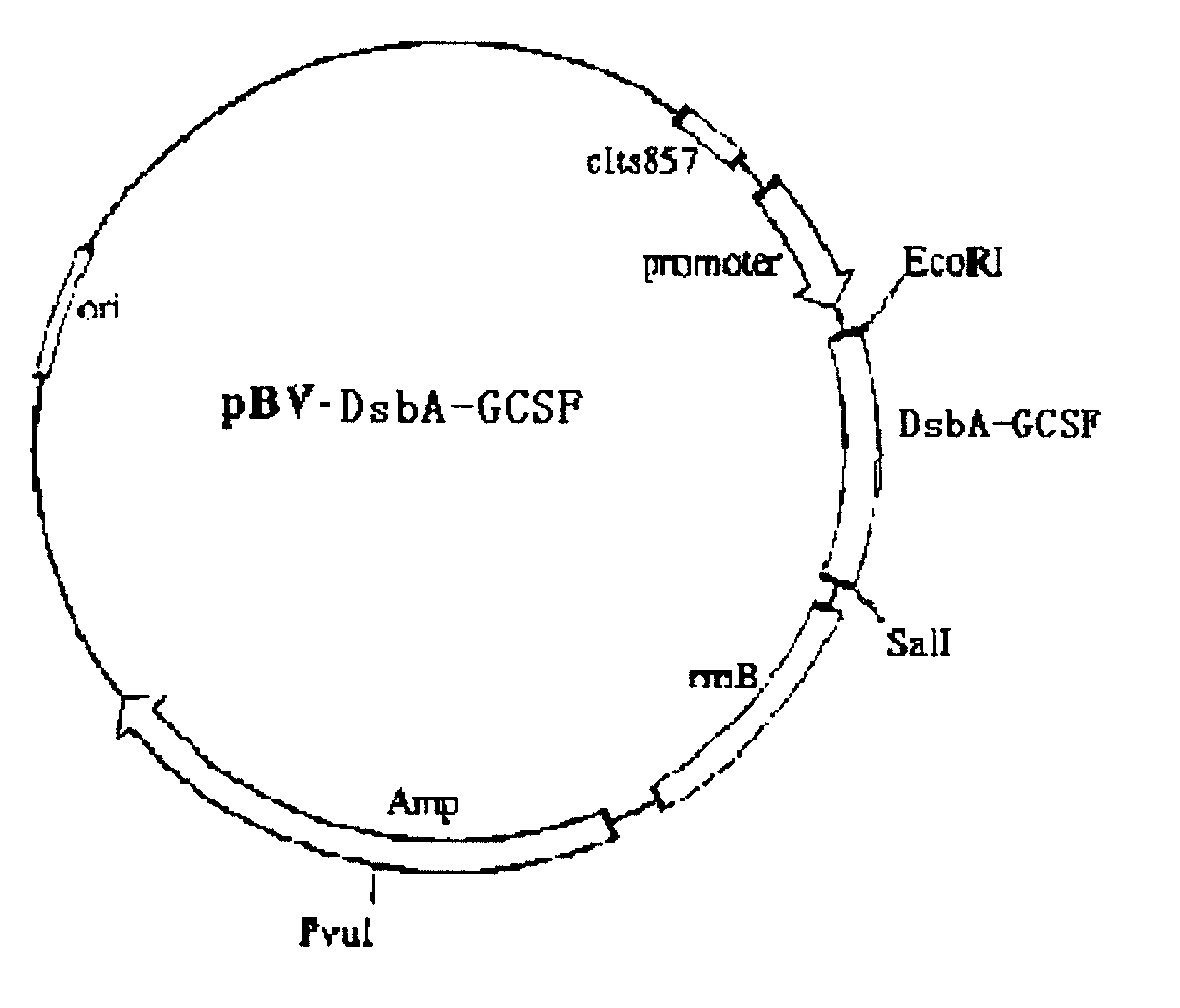
[0025] Construction of pBV-DsbA-GCSF plasmid
[0026] In this example, a plasmid was constructed containing the DsbA-GCSF sequence and the rmB termination sequence. The rmB sequence was provided by plasmid pBv220, a known expression plasmid. The heterologous protein sequence to be expressed in this plasmid is placed downstream of the PRPL promoter, which allows expression of the protein sequence located downstream of it when heat induced. Plasmid pBV220 was double cut with BamHI / SaLI to form plasmid pBV-DsbA-GCSF. The structure of the PBV-DsbA-GCSF plasmid is as follows figure 2 As shown, immediately downstream of the DsbA-GCSF sequence is the rrnB termination sequence.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com