Compounds that participate in cooperative binding and uses thereof
A technology of compounds and macrocyclic compounds, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve insurmountable problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0527] Example 1. General Fermentation and Isolation Protocol
[0528] Compounds synthesized by bacterial strains can be fermented and isolated using the following general protocol:
[0529] General Fermentation Protocol
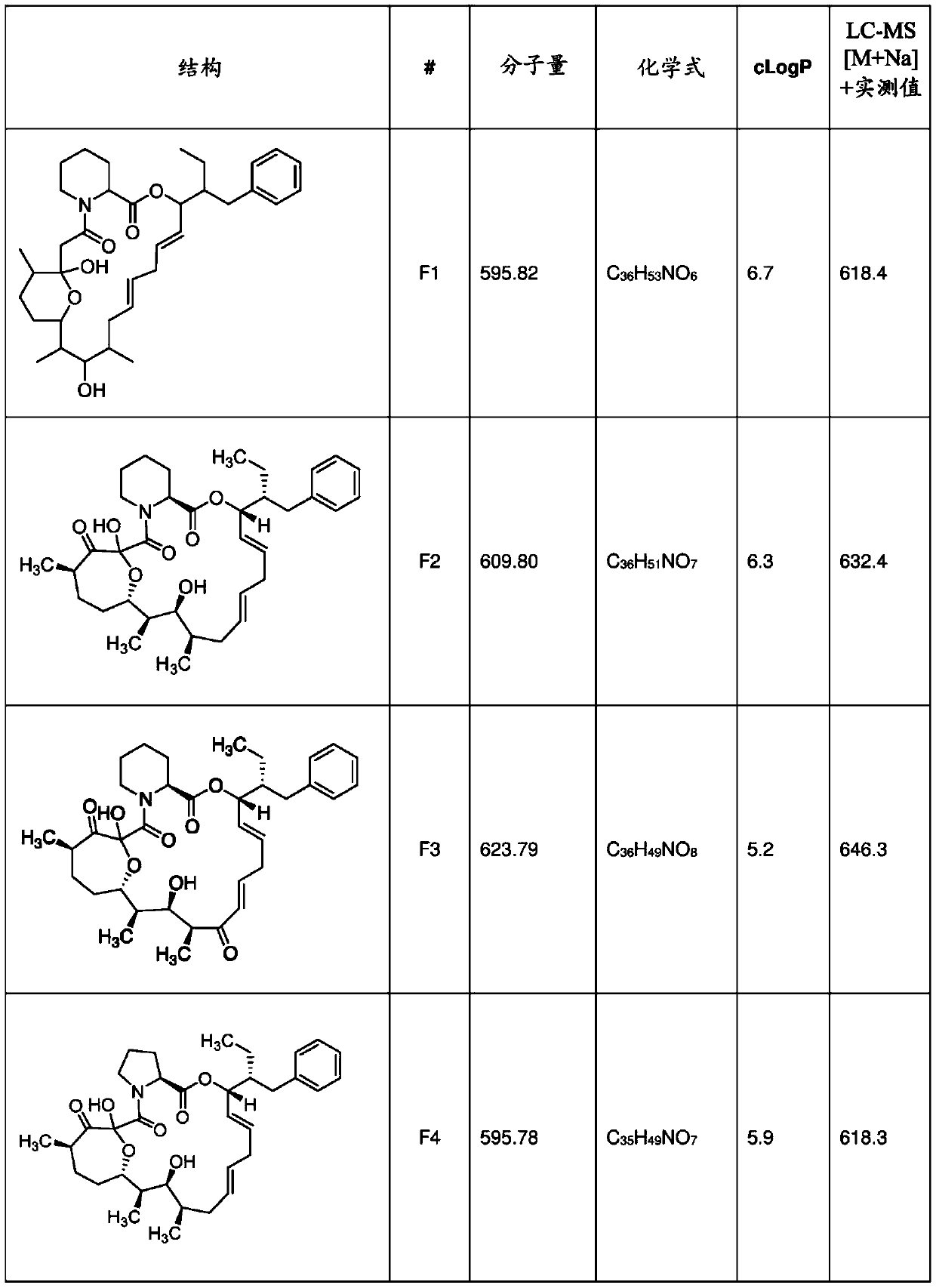
[0530] Strains: Bacterial strains such as Streptomyces malaysiensis DSM41697, other producing strains or genetically modified derivatives that produce FKBP ligands (for example: F1, F2, F3 or structurally similar compounds and their analogs) on solid culture Propagate aseptically on a substrate (eg ISP4).
[0531] Working Cell Bank: Use spores or mycelia produced from cultures grown on solid media plates for 3-14 days at 30°C to inoculate liquid cultures (eg: 40ml ATCC172 broth in a 250ml Erlenmeyer flask ). The culture was incubated at 30°C with shaking for 2-3 days. The resulting cell suspension was mixed with sterile 50% glycerol to obtain a mixture with a final concentration of 15-25% glycerol. Aliquots (approximately 1 ml) of the glycerol-mycelium ...
Embodiment 2
[0562] The separation of embodiment 2.F2 and F3
[0563] F1 (target mass 595), F2 (target mass 609) and compound 3 (target mass 623) Streptomyces malaysian fermentation broth (NRRL B-24313; ATCC BAA-13; DSM 41697; JCM 10672; KCTC9934) were produced by centrifugation in 10 L ; NBRC 16446; CGMCC 4.1900; IFO 16448). F1 and F2 are present in clarified fermentation broth and microbial pellets. The target compound in the supernatant was extracted once with EtOAc at a volume ratio (1:1, v / v). The precipitate was extracted 3 times with 1.5 L of EtOAc-MeOH (9:1, v / v), and each extraction was stirred for 1 h-1.5 h with an overhead stirrer. The organic extracts were filtered through celite. The combined filtrates were evaporated at 35°C until dryness, yielding approximately 30 g of crude extract. The residue was then dissolved in 90 mL of DCM-THF (80:20, v / v), and 60 g of silica gel was added thereto and dried under vacuum at 35°C. The dried residue / silica mixture was loaded onto a ...
Embodiment 3
[0568] The separation of embodiment 3.F22
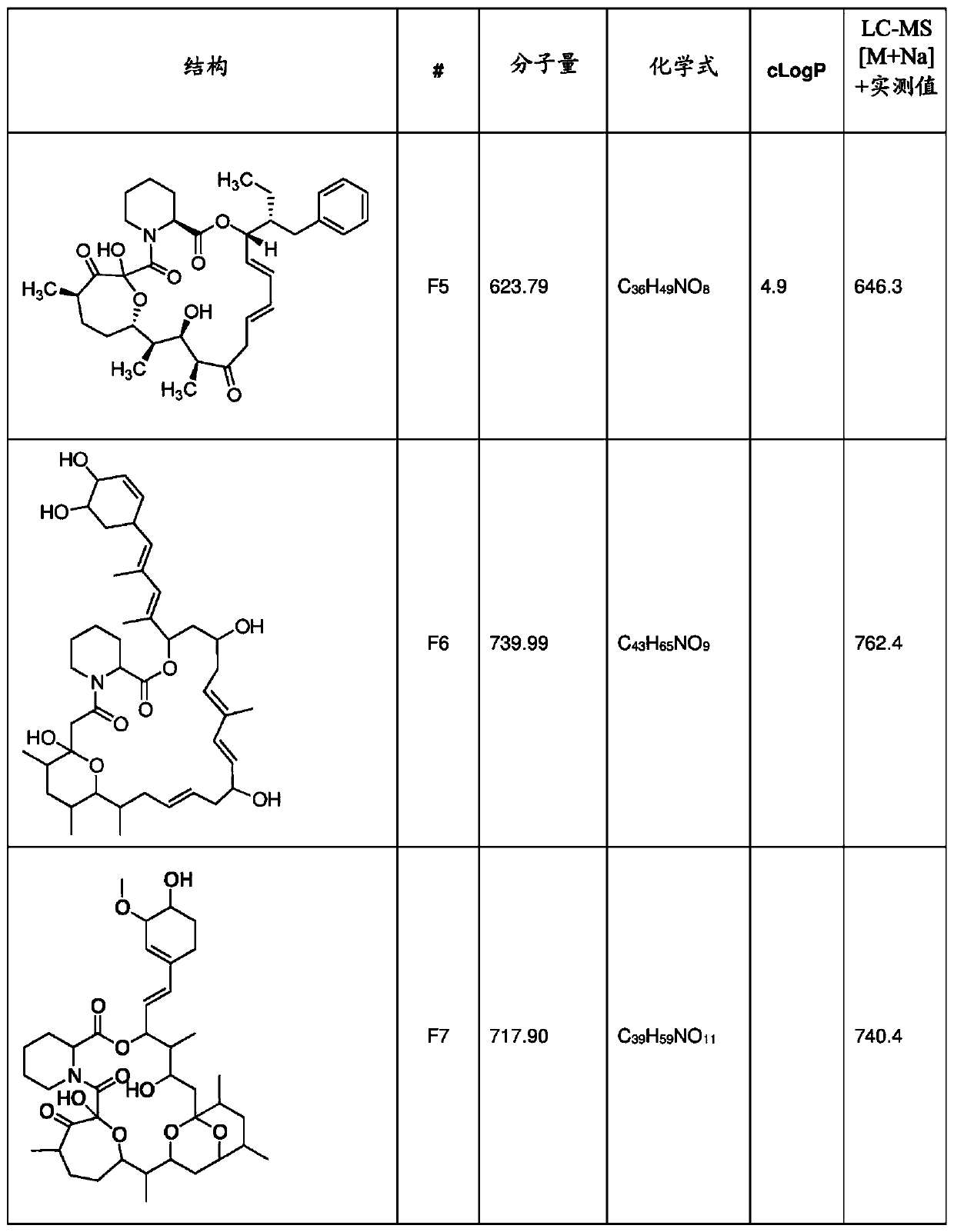
[0569] 10 L of the fermentation broth produced by the recombinant strain S1806 was centrifuged to obtain a pellet and a supernatant. The particles were extracted 3 times with 1.5LEtOAc-MeOH (9:1, v / v). The organic solvents were combined and concentrated in vacuo to obtain 1.8 g of crude extract. 2 mL of heptane-THF (4:1, v / v) was added thereto for dissolution, and then 2 g of Celite was added to obtain a dry mixture after solvent removal on a rotary evaporator at 30°C. The dried residue / celite mixture was loaded onto a 40 g RediSep silica gold cartridge for column chromatography. The compound was fractionated at 20 mL / min eluting with a linear gradient from 100% n-heptane to 40% EtOAc in heptane (v / v) over 25 min, collecting 50 mL of each fraction. F22 (target mass 607) was mainly enriched in fraction 14 identified by LC-MS analysis. Fraction 14 was then dried under vacuum at 30°C to give 17.8 mg of solid material which was furth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com