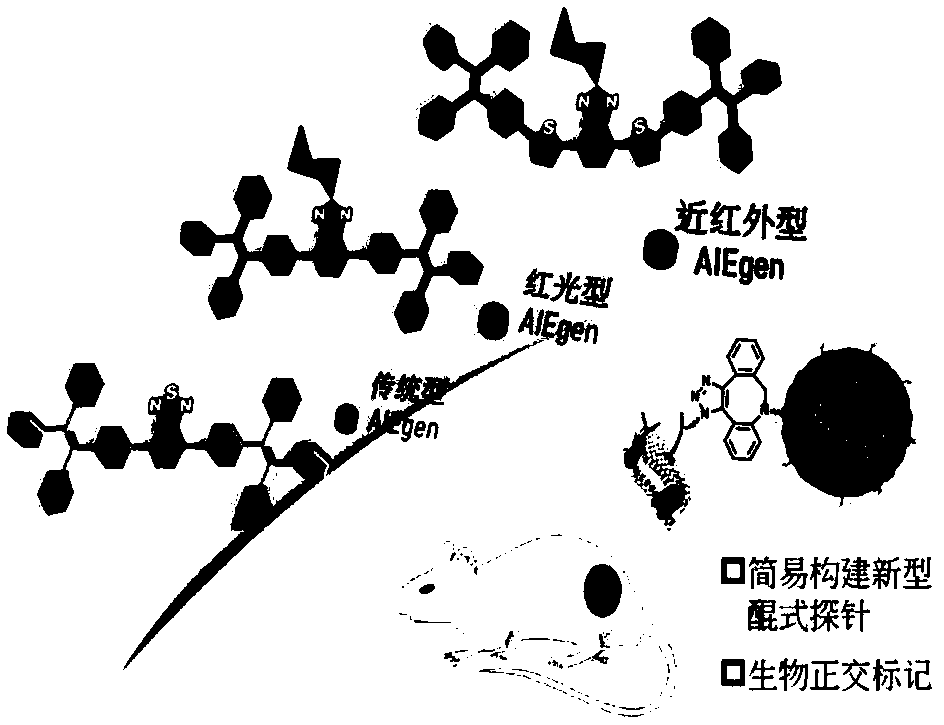
Quinone type electron withdrawing group type compound, preparation method and applications thereof
A technology of electron-withdrawing groups and electron-donating groups, which is applied in the field of compounds based on quinone-type electron-withdrawing groups and their preparation, and can solve the problems of low stability, high preparation cost of commercially labeled probes, high self-absorption, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Embodiment 1: the preparation of product
[0121] (1) Synthesis of 2TPE-BI
[0122]Add 4,7-dibromo-spiro[benzo[d]imidazole-2,1'-cyclohexane](Br-BI-Br; 1.00g, 2.91mmol), Tributyl(4-(1,2,2-triphenylvinyl)phenyl)stannane, TPE-SnBu 3 ; 3.62g, 5.82mmol), ditriphenylphosphine palladium dichloride (Pd(PPh 3 ) 2 Cl 2 ; 0.25g, 0.35mmol) and anhydrous and oxygen-free dimethylformamide (DMF; 20mL) solvent, refluxed and stirred for 24 hours. After the reaction mixture was placed in a reduced pressure environment to remove the solvent, the orange-red 2TPE-BI product (0.64 g) was obtained by column purification with a yield of 26%. 1 H NMR (400MHz, CDCl 3 ),δ(ppm):7.80-7.78(m,4H),7.23(s,2H),7.13-7.03(m,34H),1.95(m,4H),1.75-1.66(m,6H). 13 C NMR (100MHz, CDCl 3 ,δ):159.22,144.05,143.90,143.83,143.82,141.49,140.75,134.78,134.38,131.60,131.58,131.51,131.48,130.86,127.94,127.83,127.76,127.61,126.69,126.63,126.58,107.53,33.30, 25.95,25.07.HRMS(MALDI-TOF)m / z:[M] + calculated for C...
Embodiment 2
[0131] Example 2: Research on Optical and Aggregation-Induced Luminescent Properties of Products
[0132] Taking three quinone-type electron-withdrawing group fluorescent products 2TPA-BI, 2TPA-T-BI and 2TPA-2T-BI as examples to illustrate their optical properties and aggregation-induced luminescent properties. The same is true for other products, and details will not be repeated here.
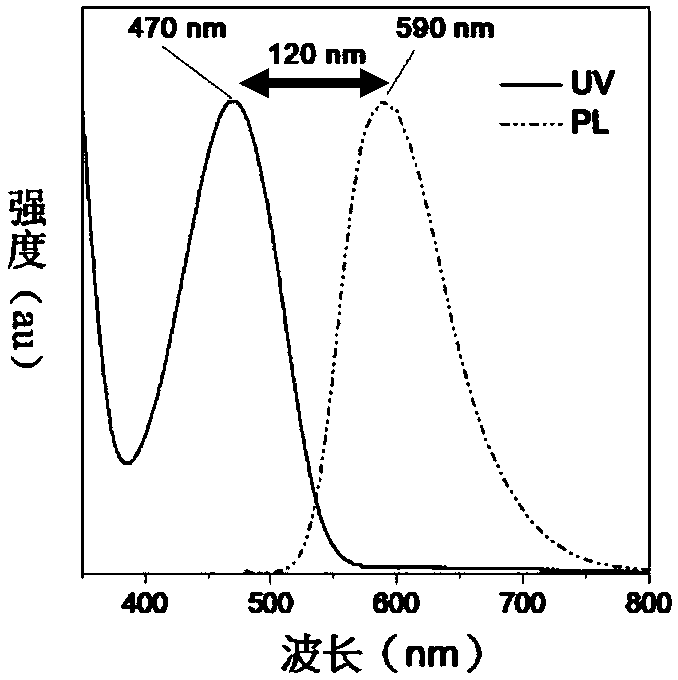
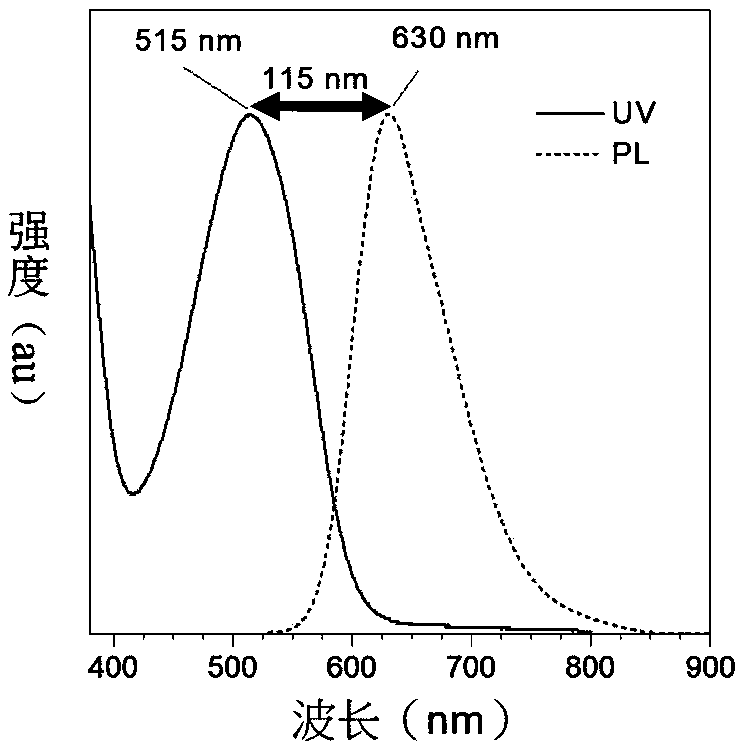
[0133] (1) Research on optical properties and thermal stability
[0134] The ultraviolet-visible (UV-vis) absorption spectrum and photoluminescence (PL) spectrum of 2TPA-BI, 2TPA-T-BI and 2TPA-2T-BI in tetrahydrofuran (THF) solution are as follows Figure 2-4 shown. The maximum absorption wavelengths of the three products in THF solution are 470, 515 and 563nm, the emission wavelengths are 590, 630 and 677nm respectively, and the Stokes shifts are 120nm (2TPA-BI), 115nm (2TPA-T -BI) and 144nm (2TPA-2T-BI), the characteristics of the large Stokes shift and long wavelength emission, as the pr...
Embodiment 3
[0145] Example 3: Application of the product in in vitro cancer cell and in vivo tumor tissue labeling and imaging
[0146] (1) Preparation of aggregation-induced emission nanoparticles (AIE dots)
[0147] like Figure 27 As shown, the 2TPE-2T-BI product that can emit near-infrared light, the amphoteric compound DSPE-PEG, and the DSPE-PEG-DBCO molecule with a bioorthogonal DBCO functional group were mixed in phosphate buffered saline (PBS ) environment for nanoprecipitation to obtain DBCO-AIEdots nanoparticles with bioorthogonal reaction capability. like Figure 28 As shown, the absorption and emission wavelengths of DBCO-AIEdots nanoprobes extend from 572 and 710 nm to the near-infrared region, respectively, and its maximum Stokes shift is 138 nm. In addition, if Figure 29-30 As shown, the particle size of DBCO-AIEdots nanoparticles is between 85 nm, which is quite suitable for blood circulation process of in vivo labeling and imaging.
[0148] (2) Cell culture
[0149...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com