Method for preparing and purifying polypeptides
A purification method and polypeptide technology, applied in the field of medicine, can solve the problems such as no peptide amino acid oxide, no targeted research on methionine oxidation impurities, and no effective detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The preparation and purification of embodiment 1 ularitide
[0082] (1) Dissolution and oxidation of the crude product of ularitide linear peptide
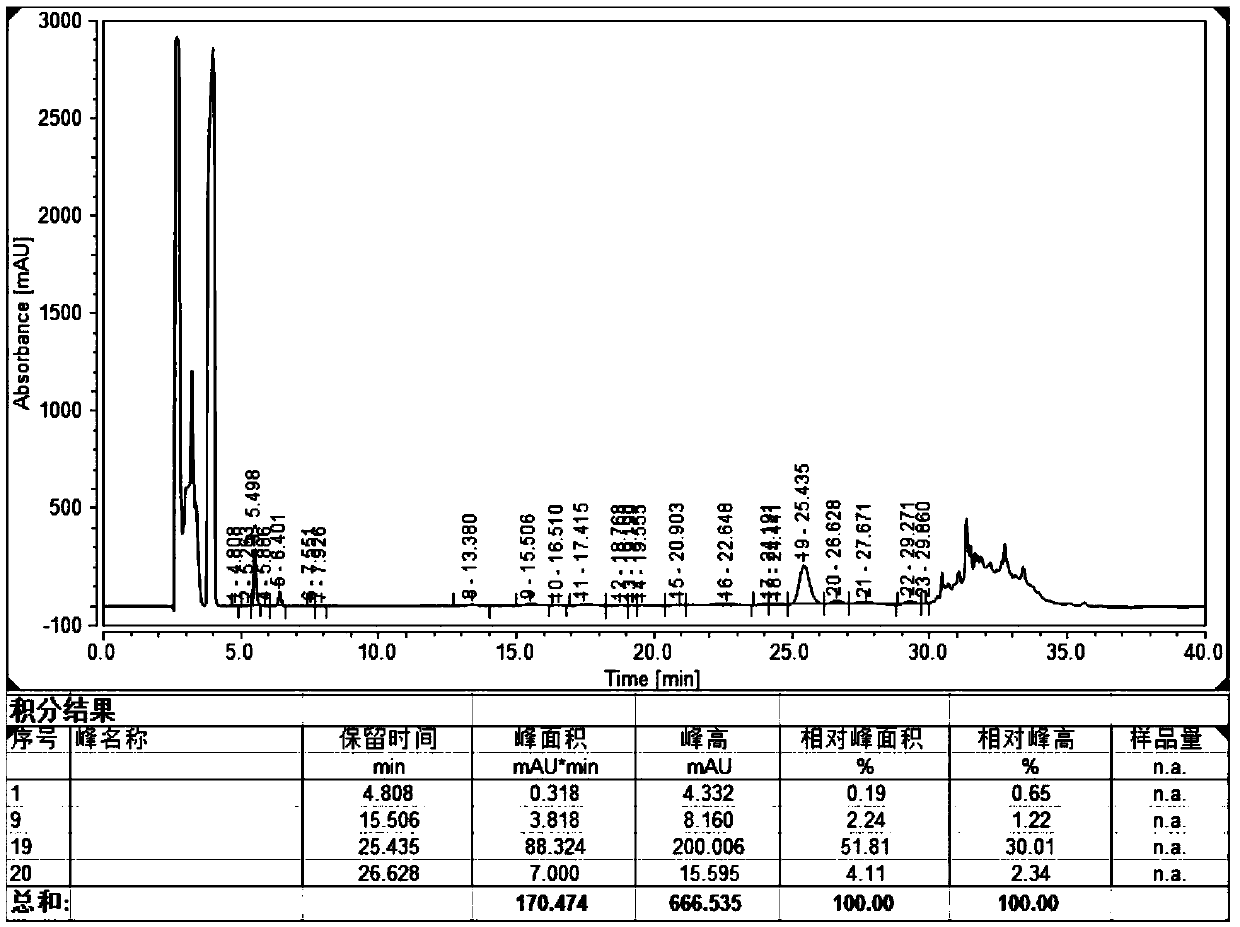
[0083]Take 10.0 g of the crude linear peptide, stir and dissolve it with 10.0 L, pH 8.0, 1 mol / L ammonium acetate aqueous solution, and oxidize it with stirring air at 25°C. After 6 hours of oxidation, the content of cysteine residues is detected to be 0, and the oxidation is stopped. ; HPLC detects the oxidation solution, and the HPLC purity of ularitide is 51.81%, and the methionine oxidation impurity (relative retention time=0.61) is 2.24% ( figure 1 ).
[0084] (2) Concentration of oxidizing solution
[0085] Concentrate 10.0 L of the ularitide oxidation solution obtained in step (1) by nanofiltration, stop the concentration when the volume reaches 1.0-1.2 L, and adjust the pH of the concentrated solution to 6.0 with acetic acid for later use.
[0086] (3) Purification by high performance liquid chromatography
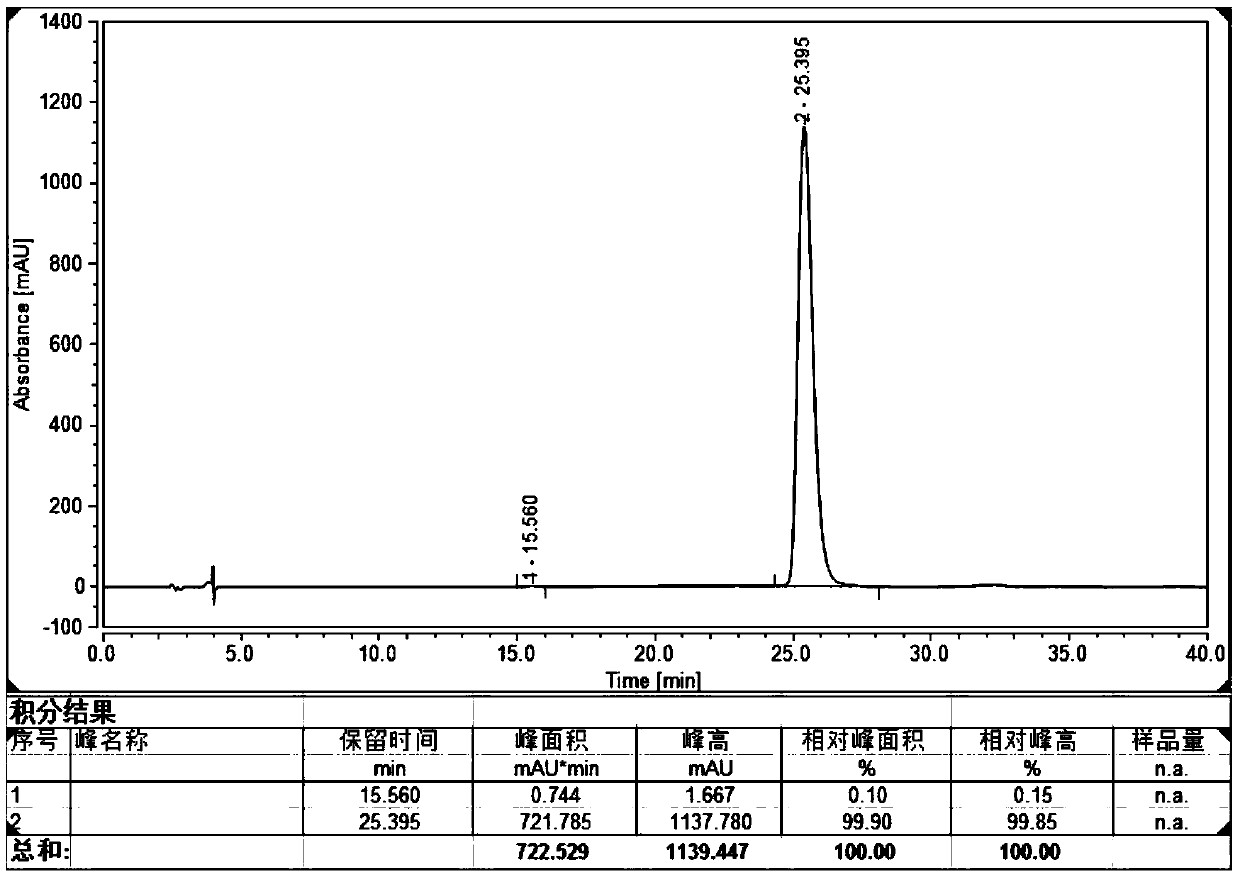
[...
Embodiment 2
[0094] The preparation and purification of embodiment 2 ularitide
[0095] (1) Dissolution and oxidation of the crude product of ularitide linear peptide
[0096] Take 10.0 g of crude linear peptide, stir and dissolve it with 10.0 L, pH 6.0, 1.5 mol / L aqueous solution of potassium dihydrogen phosphate, and oxidize it with stirring air at 20°C. After 8 hours of oxidation, the content of cysteine residues is detected to be 0. , stop oxidation; HPLC detection of oxidation solution, HPLC purity of ularitide is 52.12%, methionine oxidation impurity is 2.15%.
[0097] (2) Concentration of oxidizing solution
[0098] 10.0 L of the ularitide oxidation solution obtained in step (1) was applied to a D152 cation exchange resin column, desalted and eluted with 0.1 N hydrochloric acid aqueous solution, and the elution volume was 1.0-1.2 L for later use.
[0099] (3) Purification by high performance liquid chromatography
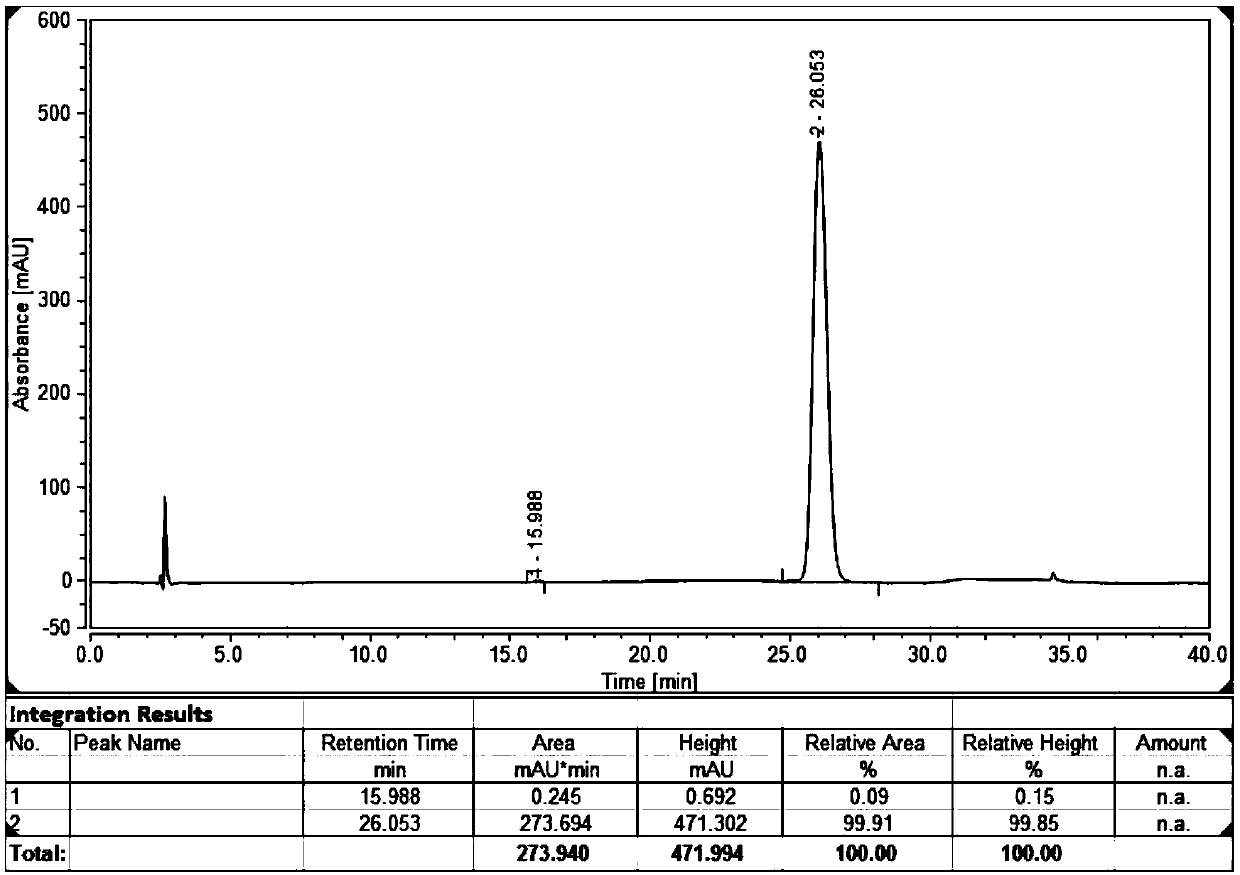
[0100] DAC50 column is used, the water circulation outside the ...
Embodiment 3
[0107] The preparation and purification of embodiment 3 ularitide
[0108] (1) Dissolution and oxidation of the crude product of ularitide linear peptide
[0109] Take 10.0 g of the crude linear peptide, stir and dissolve it with 10.0 L, pH 6.5, 5.0 mol / L ammonium chloride aqueous solution, and oxidize it in air with stirring at 30 ° C. After 5 h of oxidation, the content of cysteine residues is detected to be 0. Oxidation was stopped; the oxidized solution was detected by HPLC, and the HPLC purity of ularitide was 51.78%, and the methionine oxidation impurity was 2.18%.
[0110] (2) Concentration of oxidizing solution
[0111] Concentrate 10.0 L of the ularitide oxidation solution obtained in step (1) by nanofiltration, stop the concentration when the volume reaches 1.0-1.2 L, and adjust the pH of the elution concentrate to 6.0 with dilute hydrochloric acid for later use.
[0112] (3) Purification by high performance liquid chromatography
[0113] DAC50 column is used, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com