Preparation method of sulfur-doped titania nanofibers
A nanofiber and titanium dioxide technology, applied in the field of material chemistry, can solve the problems of low utilization rate of sulfur cathode active material and poor cycle performance, and achieve the effect of uniform particle size and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
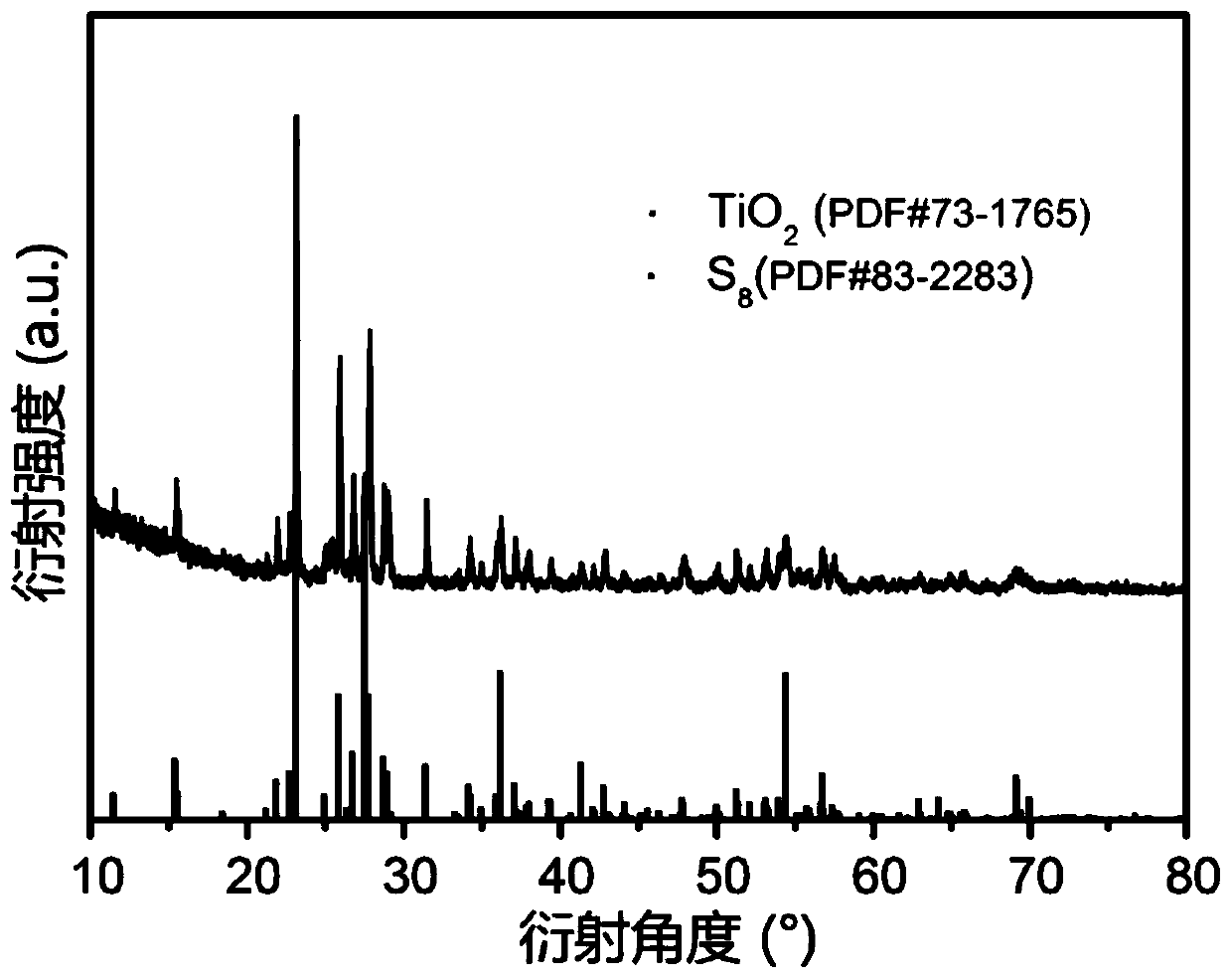
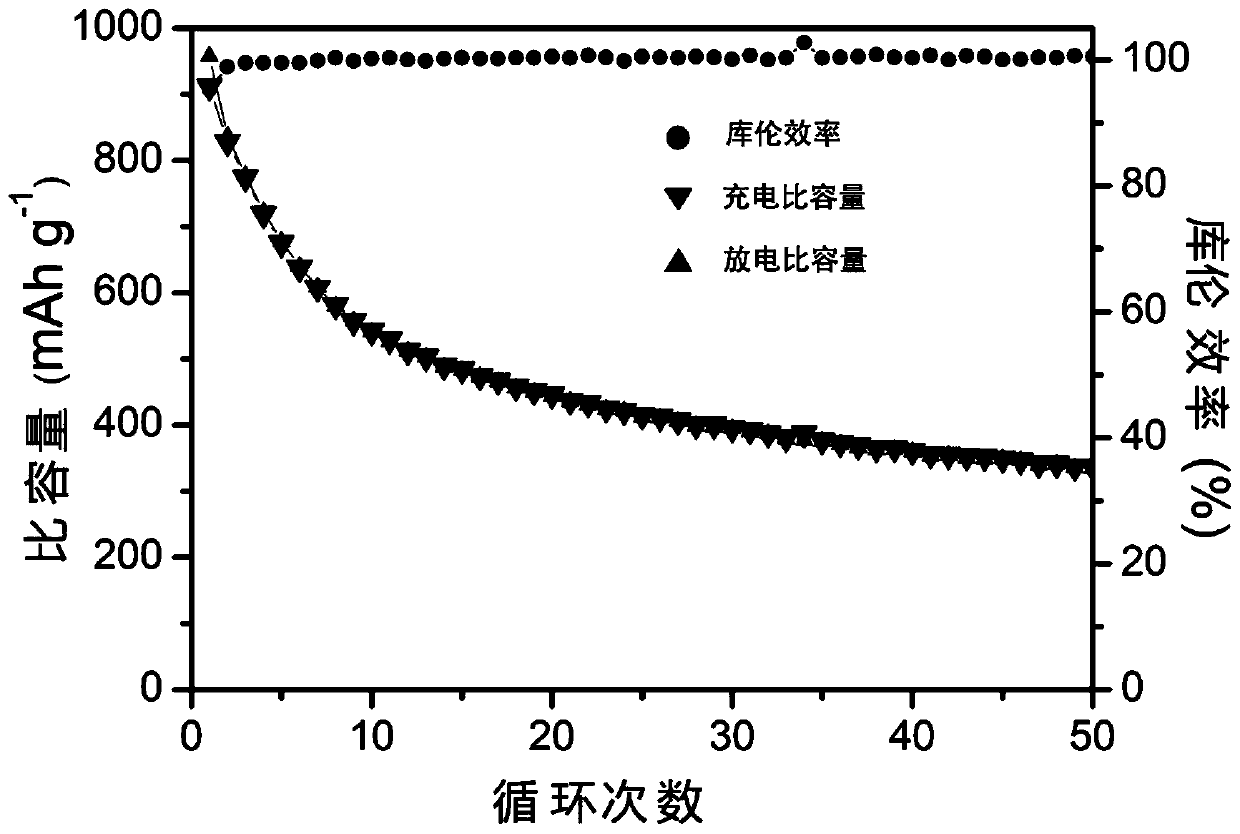
[0024] 2.0g tetrabutyl titanate (C 16 h 36 o 4 Ti) was dissolved in a mixed solvent of 12mL of DMF, 12mL of ethanol and 6.0mL of acetic acid, added 2.0g of PVP, stirred for 3h to form solution A; 2.0g of PVP was dissolved in 12mL of DMF, 12mL of ethanol and 6.0mL of In the mixed solvent of acetic acid, stir for 3h to form solution B; put the two solutions A and B at a voltage of 17kV, a receiving distance of 15cm and 0.6mL h -1 Coaxial electrospinning was carried out at a flow rate of 100°C, solution B was the inner layer solution, and solution A was the outer layer solution; the obtained electrospun product was placed in an oven and dried at 100°C for 12 hours; the dried electrospun product was The product was transferred to a muffle furnace and sintered at 750°C for 5h to obtain TiO 2 nanotubes; 0.2 g of TiO 2 Mix the nanotubes with 0.3g of sublimed sulfur, put them into a closed container, and carry out melt sulfurization at 155°C; put 0.2g of TiO after sulfurization 2...
Embodiment 2
[0026] 2.0g tetrabutyl titanate (C 16 h 36 o 4 Ti) was dissolved in a mixed solvent of 12mL of DMF, 12mL of ethanol and 6.0mL of acetic acid, added 2.0g of PVP, stirred for 3h to form solution A; 2.0g of PVP was dissolved in 12mL of DMF, 12mL of ethanol and 6.0mL of In the mixed solvent of acetic acid, stirred for 3h to form solution B; the two solutions A and B were mixed at a voltage of 19kV, a receiving distance of 20cm and 0.6mL h -1 Coaxial electrospinning was carried out at a flow rate of 100°C, solution B was the inner layer solution, and solution A was the outer layer solution; the obtained electrospun product was placed in an oven and dried at 100°C for 12 hours; the dried electrospun product was The product was transferred to a muffle furnace and sintered at 900°C for 5h to obtain TiO 2 nanotubes; 0.2 g of TiO 2 Mix the nanotubes with 0.3g of sublimed sulfur, put them into a closed container, and carry out melt sulfurization at 155°C; put 0.2g of TiO after sulfur...
Embodiment 3
[0028] 2.0g tetrabutyl titanate (C 16 h 36 o 4 Ti) was dissolved in a mixed solvent of 12mL of DMF, 12mL of ethanol and 6.0mL of acetic acid, added 2.0g of PVP, stirred for 3h to form solution A; 2.0g of PVP was dissolved in 12mL of DMF, 12mL of ethanol and 6.0mL of In the mixed solvent of acetic acid, stir for 3h to form solution B; put the two solutions A and B at a voltage of 18kV, a receiving distance of 19cm and 0.6mL h -1 Coaxial electrospinning was carried out at a flow rate of 100°C, solution B was the inner layer solution, and solution A was the outer layer solution; the obtained electrospun product was placed in an oven and dried at 100°C for 12 hours; the dried electrospun product was The product was transferred to a muffle furnace and sintered at 850°C for 5h to obtain TiO 2 nanotubes; 0.2 g of TiO 2 Mix the nanotubes with 0.3g of sublimed sulfur, put them into a closed container, and carry out melt sulfurization at 155°C; put 0.2g of TiO after sulfurization 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com