Method for separating related substances from rivaroxaban
A separation method and technology for related substances, applied in the field of pharmaceutical analysis, can solve the problems of complicated gradient programming, and achieve the effects of good peak shape, good resolution and simple mobile phase composition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of the test sample: Take 5 rivaroxaban tablets and place them in a 100ml measuring bottle, add an appropriate amount of diluent (mobile phase A:mobile phase B=40:60) about 70ml, ultrasonicate for 15min to dissolve, cool to room temperature, and dilute with Dilute the solution to the mark, shake well, filter, and take the continued filtration solution.
[0038] Mixed solution: Weigh about 25mg of rivaroxaban, put it in a 25ml measuring bottle, add an appropriate amount of diluent to dissolve, add acetooxamide, dioxalamide-urea, dechlorinated compound, 4,5-dichloro compound, oxygen Add appropriate amount of impurity solutions such as substituted phthalimide, diamide, triamide, intermediate 1, intermediate 3, impurity K and impurity M, and dilute to the mark with diluent to obtain the product.
[0039] Chromatographic conditions: mobile phase A is 0.01mol / L phosphoric acid solution, mobile phase B is acetonitrile, pH value of mobile phase A is 1.8, flow rate is...
Embodiment 2
[0046] Need testing solution and mixed solution preparation are consistent with embodiment 1.
[0047] Chromatographic conditions: mobile phase A is 0.01mol / L phosphoric acid solution, mobile phase B is acetonitrile, pH value of mobile phase A is 1.8, flow rate is 1.0ml / min, column temperature is 45°C, wavelength: 250nm;
[0048] time (min) A B 0 95% 5% 3 95% 5% 20 49% 51% 25 95% 5% 30 95% 5%
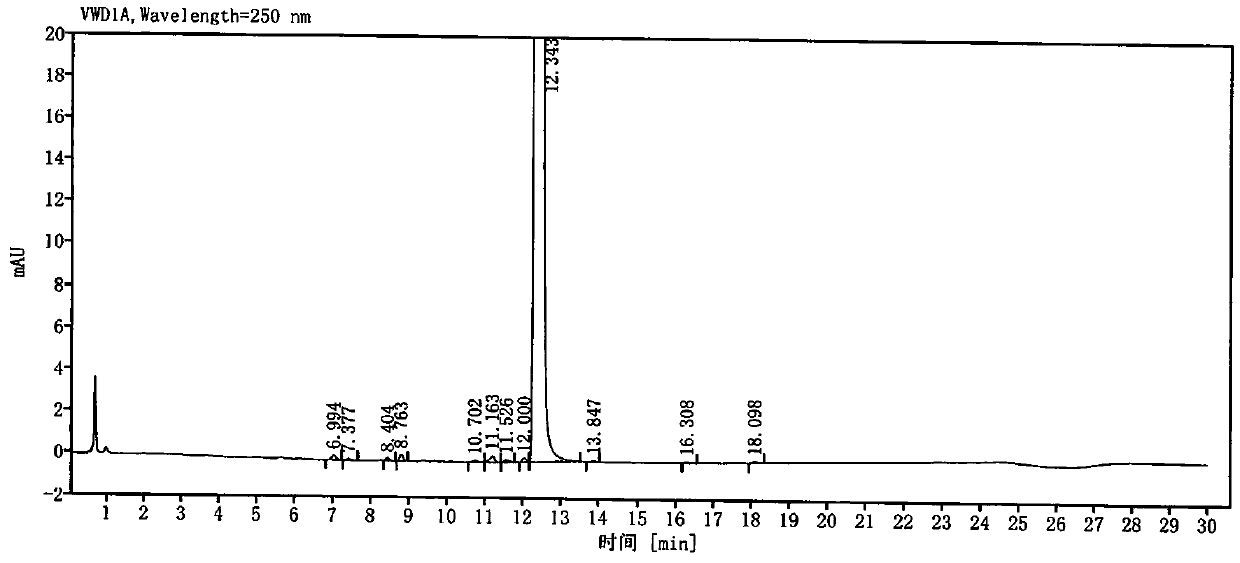
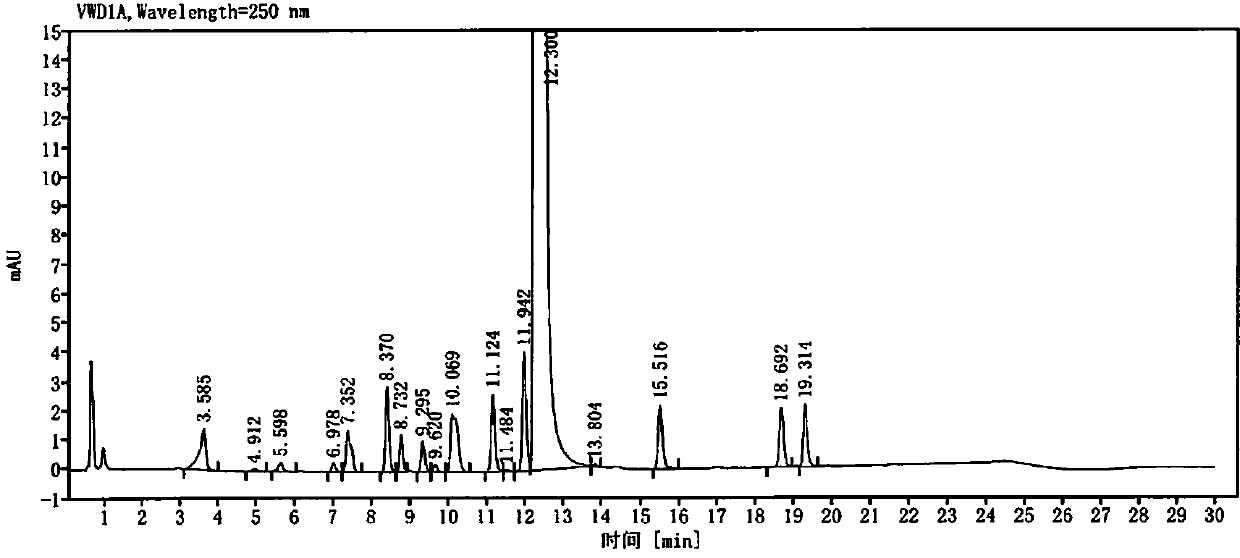
[0049] Get need testing solution, each 5ul of mixed solution inject high performance liquid chromatograph, and record chromatogram, obtain attached image 3 and 4 .
[0050] peak number keep time Peak height Peak area Tailing factor Resolution USP Theoretical plate number USP 1 10.122 0.272 1.552 1.068 / 71558.3 2 11.202 0.169 0.807 0.840 7.379 100504.5 3 11.323 0.393 1.899 1.134 0.887 123007.9 4 12.860 0.086 0.795 1.060 7.869 38713.0 5 13.184 0.343 2.603 0.847 1.506 98...
Embodiment 3
[0054] Need testing solution and mixed solution preparation are consistent with embodiment 1.
[0055] Chromatographic conditions: mobile phase A is 0.01mol / L phosphoric acid solution, mobile phase B is acetonitrile, pH value of mobile phase A is 2.0, flow rate is 1.0ml / min, column temperature is 45°C, wavelength: 250nm;
[0056] time (min) A B 0 92% 8% 12 65% 35% 20 49% 51% 24 92% 8% 30 92% 8%
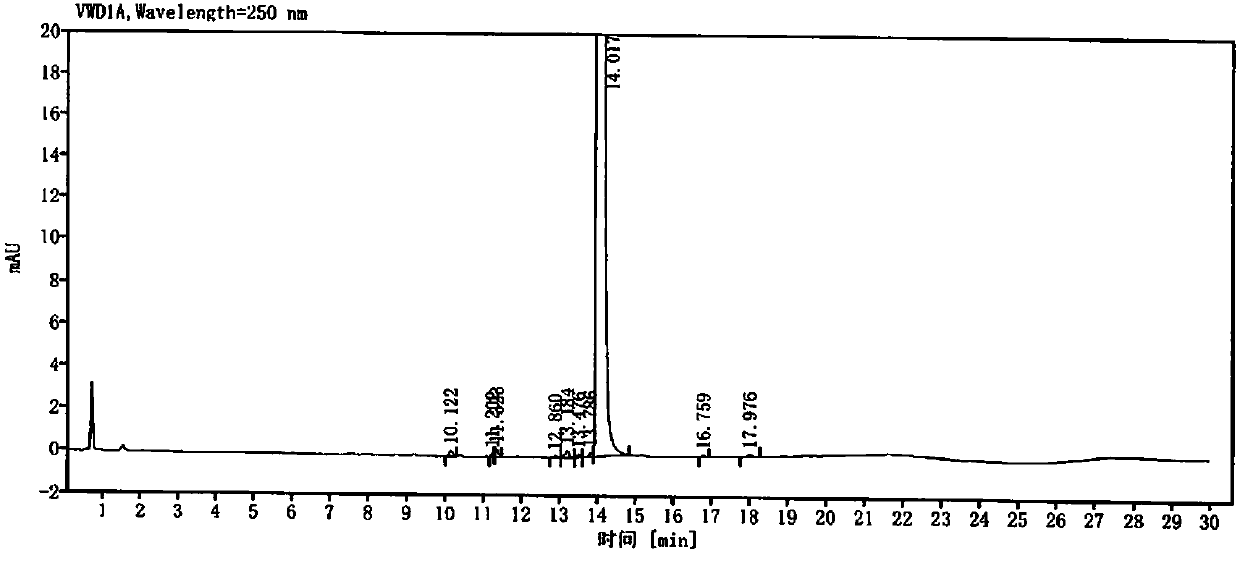
[0057] Get need testing solution, each 5ul of mixed solution inject high performance liquid chromatograph, and record chromatogram, obtain attached Figure 5 and 6 .
[0058] peak number keep time Peak height Peak area Tailing factor Resolution USP Theoretical plate number USP 1 6.546 0.247 1.503 1.057 / 25413.0 2 7.705 0.154 0.853 0.982 7.403 43229.2 3 7.925 0.346 1.797 1.099 1.527 51313.6 4 9.657 0.076 0.710 1.296 8.937 24265.9 5 10.042 0.326 2.534 0.842 1.762 44299...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com