Compositions containing sodium pyruvate and application thereof
A technology of sodium pyruvate and composition, which is applied in the fields of drug combination, food science, anhydride/acid/halide active ingredients, etc., can solve the problems of no effective physiological effect, long-term tolerance, and clinical inability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0086] Reagent preparation:
[0087] Drug group: 0.35% sodium pyruvate, 0.2% sodium chloride, 0.15% potassium chloride and 1.35% glucose formula aqueous solution, instead of conventional animal laboratory drinking water;
[0088] Normal group: routine laboratory animal drinking water;
[0089] Control group: 1.0% sodium pyruvate aqueous solution, instead of drinking water in the conventional animal laboratory.
experiment Embodiment 1
[0091] Experimental animals:
[0092] Normal group: C57BLKS / J normal untreated mice: drinking regular laboratory drinking water;
[0093] Control group: C57BLKS / J diabetes db / db untreated mice (conventional human type II diabetes animal experimental model): drinking regular laboratory drinking water;
[0094] 1% Pyr: Drink db / db mice containing pure 1% sodium pyruvate distilled water to replace regular laboratory drinking water;
[0095] Drug group: Drinking an aqueous solution containing the sodium pyruvate composition of the present invention to replace conventional laboratory drinking water db / db mice, which contains 0.35% sodium pyruvate, 0.2% sodium chloride, 0.15% chlorination Potassium and 1.35% glucose.
[0096] experimental method:
[0097] The above randomly selected diabetic mice (10 weeks old) were divided into three groups, and the normal mouse group of the same strain drank different experimental water respectively, and observed and recorded under the same experimental con...
experiment Embodiment 2
[0133] Cell experiment
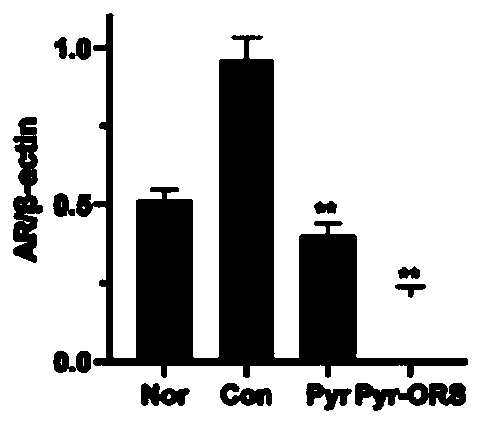
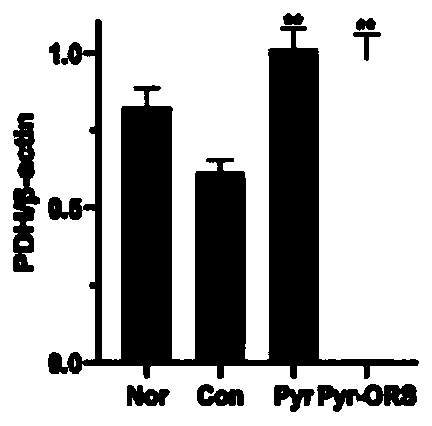
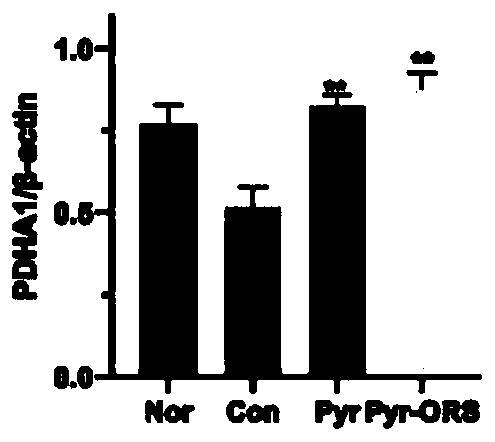
[0134] Human renal tubular epithelial cell line: HK-2 cells are combined with 0.5 mmol / L sodium pyruvate (5 times the normal serum level) under high glucose (30mmol / L) and normal levels (5mmol / L) conditions: previous experiments It has been confirmed that oral administration of the above sodium pyruvate composition can increase the level of pyruvate by more than 5 times in animal plasma[15]) and incubate together for 3 days. The determination method by routine experiment: Western Blot method to determine AR PDH / PDHA1 ( The main active components in PDH) and pyruvate dehydrogenase kinase (PDK) activity, the results showed the same as the above in vivo experimental results: inhibition of enhanced AR and PDK, restored PDH and PDHA1 activity.
[0135] It is worth noting that PDK in human cells will be stimulated and enhanced by many other pathogenic factors besides high glucose, such as hypoxia, oxidative stress, tumor or aging; PDK is the kinase of PDH, which ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com