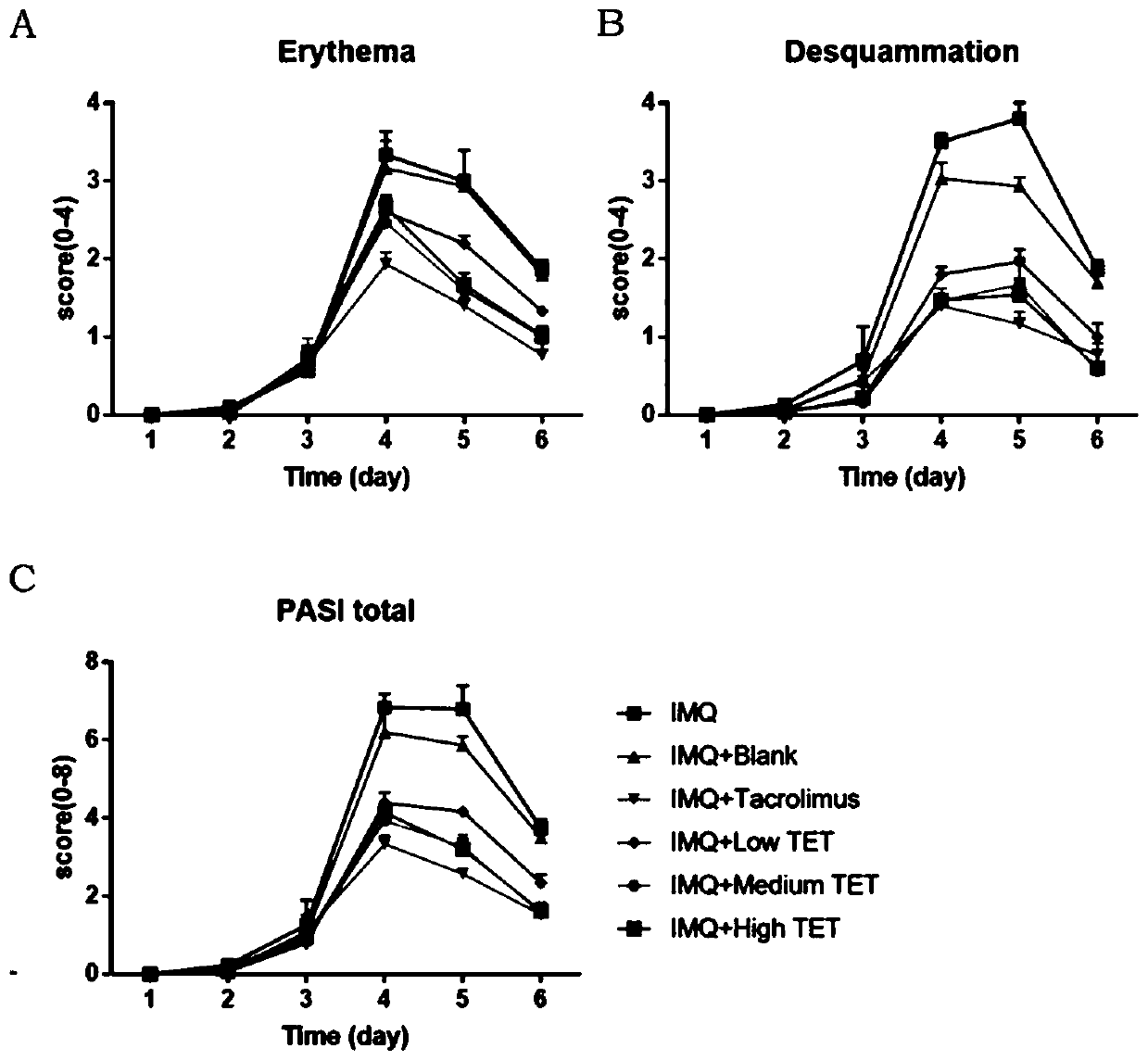
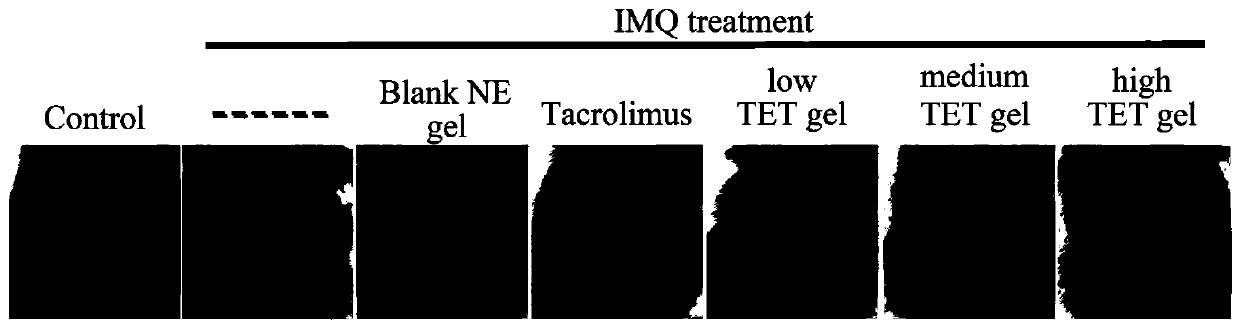
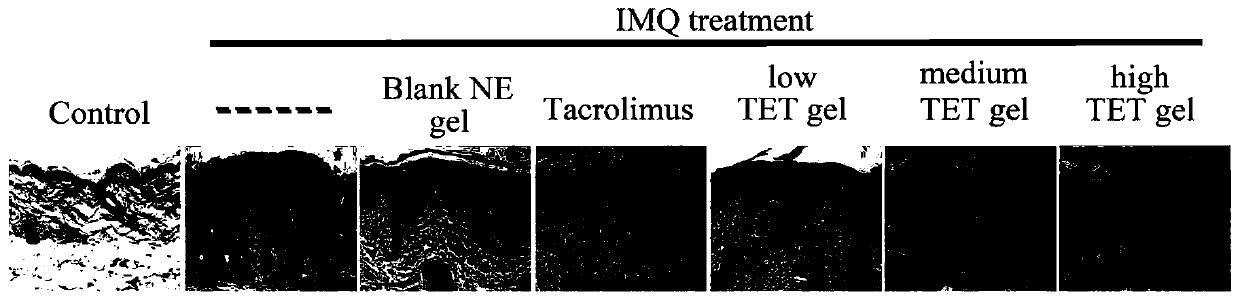
Tetrandrine composition, preparation method of tetrandrine composition, external tetrandrine preparation, and preparation method and application of external tetrandrine preparation
A technology of tetrandrine and topical preparations, applied in the field of preparation of tetrandrine topical preparations and tetrandrine compositions, which can solve problems such as low bioavailability, poor targeting, and renal toxicity, and achieve good therapeutic effects , Solubility improvement, and the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The embodiment of the present invention also provides a preparation method of a tetrandrine composition, comprising: mixing an oil phase, an emulsifier and an emulsifier, and then mixing with a tetrandrine and a water phase to form the tetrandrine composition.
[0061] Specifically, the oil phase, emulsifier and co-emulsion agent are uniformly mixed at a temperature of 40-60° C., and then tetrandrine is added to the above mixture and stirred until the tetrandrine is dissolved.
[0062] Then heat the water phase to 40-60°C, then add the water phase to the mixture of oil phase, emulsifier, co-emulsion agent and tetrandrine, stop heating, and continue stirring to room temperature (generally 25°C).
[0063] The embodiment of the present invention also provides a preparation method of an external preparation of tetrandrine. When the external preparation of tetrandrine is a gel, the preparation method comprises: mixing the above-mentioned tetrandrine composition with the swoll...
Embodiment 1
[0071] This embodiment provides a tetrandrine composition, which includes 0.015 g of the tetrandrine composition, 1 g of Labrafil M1994CS, 0.5 g of PEG4000, 151 g of SolutolHS, and 7.5 g of ultrapure water.
[0072] The present embodiment also provides a gel containing the above tetrandrine composition, which contains 1 gram of LabrafilM1994CS, 0.5 grams of PEG4000, 151 grams of SolutolHS, 7.5 grams of ultrapure water and carbomer 974N, wherein carbomer is present in the gel The mass content in is 0.8%, and the pH of the gel is 7.
[0073] This embodiment also provides a preparation method of tetrandrine composition, comprising:
[0074] Mix LabrafilM1994CS, SolutolHS15 and PEG400 evenly in a beaker with magnetic stirring, in a constant temperature water bath at 50°C, add tetrandrine powder, and continue stirring until the powder dissolves. The prepared ultrapure water was preheated to 50°C, added dropwise to the above mixture, the heating in the water bath was stopped, and t...
Embodiment 2- Embodiment 3
[0079] The gel is prepared according to the preparation method of Example 1. The consumption of each substance in the tetrandrine composition is the same as in Example 1, the difference is that the mass content of Carbomer 974N in the gel is different, and in Example 2, Carbomer The mass content of 974N is 1.2%, and the mass content of Carbomer 974N in Example 3 is 1.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com