Kit for detecting five pathogens of TORCH and application of kit
A pathogen and kit technology, applied in the field of genetic engineering, can solve the problems of poor sensitivity and specificity, long operation time, complex reagents, etc., and achieve the effects of simple operation, good specificity, and accurate and sensitive detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] TORCH detection of five pathogens:
[0040] (1) Extraction of genomic DNA
[0041] To collect the samples of the subject to be tested, a commercially available virus genome DNA / RNA extraction kit was used, and the specific operation process was carried out according to the instructions.
[0042] (2) Primer design
[0043] The corresponding sequences of toxoplasma gondii (TOX), rubella virus (RV), human cytomegalovirus (CMV), herpes simplex virus type I (HSV1) and herpes simplex virus type II (HSV2) were obtained from GenBank, and amplification primers were designed to detect needles to ensure that each pair of primers and probes can amplify the corresponding pathogen and not cause non-specific amplification with other pathogens.
[0044] Table 1 TORCH amplification primers and probes for five pathogens
[0045]
[0046] (3) Establishment of multiplex PCR method
[0047] Using the kit provided in this application, the reaction system is 20uL, specifically: 2uL of nu...
Embodiment 2
[0057] Specificity experiments for primer-probe sets
[0058] Use TORCH five pathogenic plasmids and known pathogen samples to do cross experiments to verify its specificity.
[0059] Add the corresponding primer probes of 5 kinds of TORCH pathogens to the wells of the same row of 96-well plate, and add the positive plasmid templates of the same 5 TORCH pathogens and samples of known pathogens to the wells of the same column. The samples of known pathogens include influenza virus A (FluA), Influenza Virus B (FluB), Coronavirus (OC43), Boca Virus (HBOV), Parainfluenza Virus 1 (HPIV1), Parainfluenza Virus 3 (HPIV3), Rhinovirus (HRV), Pneumonia Mycoplasma (MP) and Chlamydia pneumoniae (CP) ensure that each primer probe has an independent contact opportunity with any pathogen. Through such a cross experiment, the CT values are counted to verify the specificity of the method of the present invention.
[0060] The multiple fluorescent PCR reaction system is 20uL, specifically: 2u...
Embodiment 3
[0064] Sensitivity experiment
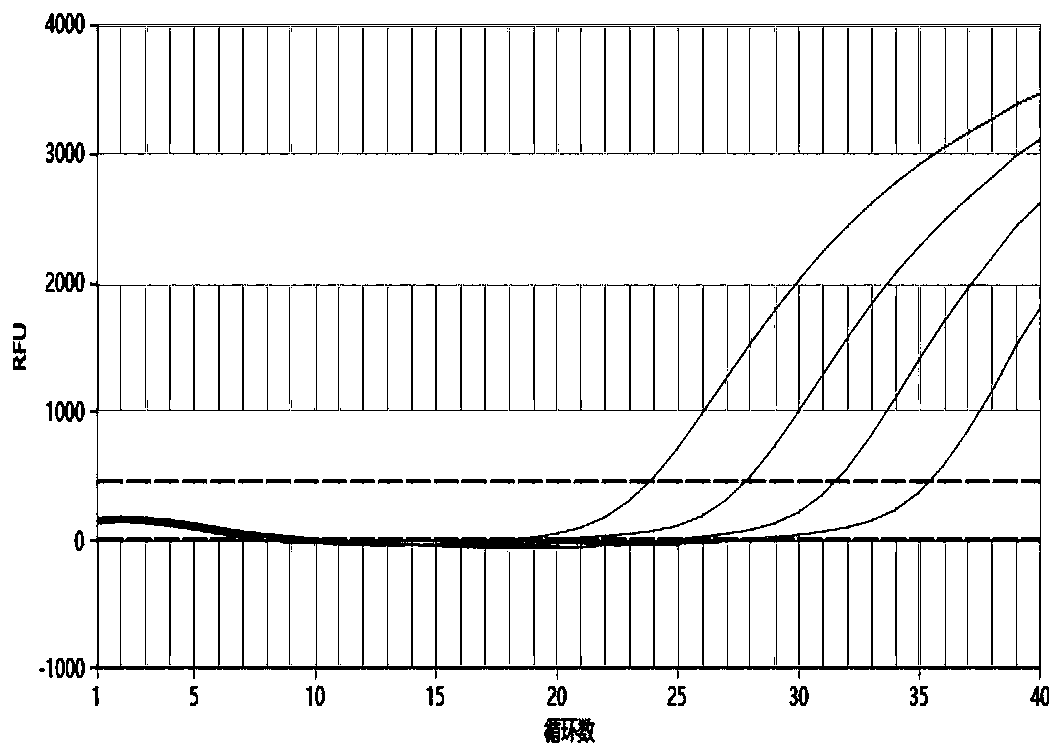
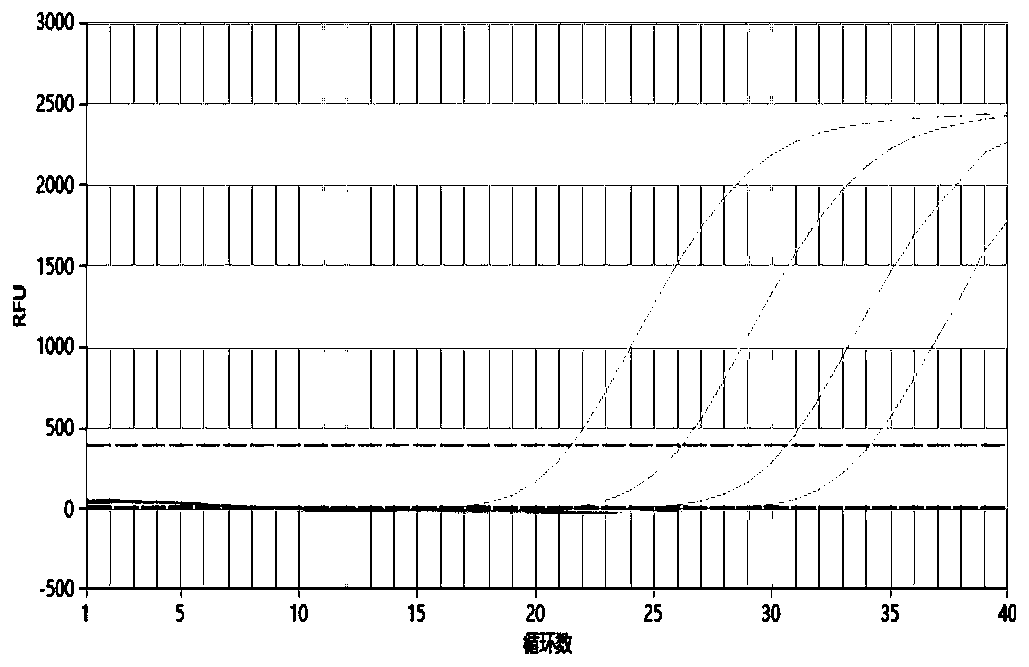
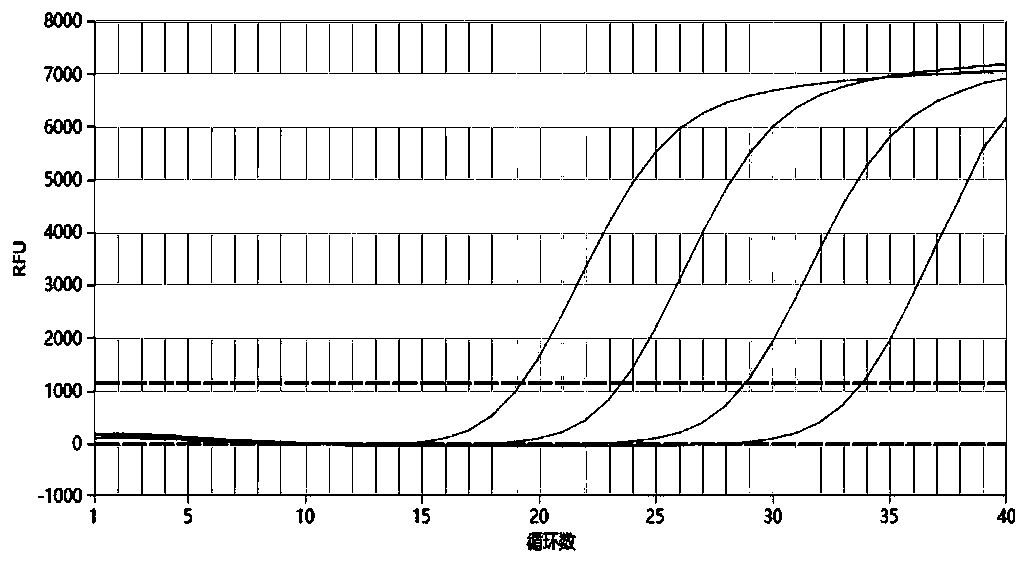
[0065] The self-made 5 kinds of TORCH pathogen positive plasmid samples were diluted tenfold, 10 2 -10 5 copy / ul, using the kit provided by this patent to amplify the above-mentioned diluted samples respectively, the detection sensitivity results of the five TORCH pathogens are as follows Figure 1-5 As shown, their R 2 The values are 0.999 (TOX), 0.996 (RV), 0.998 (CMV), 0.998 (HSV1) and 0.991 (HSV2), indicating that the linear relationship is good and the sensitivity can reach 100copies / ul.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com